Abstract
Mesenchymal stem cells (MSCs) are multipotent stem cells characterized by their robust proliferative capacity, homing ability, differentiation potential, and low immunogenicity in vitro. MSCs can be isolated from a variety of tissues, primarily including but not limited to bone marrow, adipose tissue, umbilical cord, placenta, and dental pulp. Although there have been a large number of clinical studies on the treatment of diseases by MSCs and MSCs-derived exosomes (MSCs-EXO), the large-scale clinical application of MSCs and MSCs-EXO have been limited due to the heterogeneity of the results among various studies. This review provides a detailed description of the classification and characterization of MSCs and MSCs-EXO, as well as their extraction methods. Furthermore, this review elaborates on three key mechanisms of MSCs and MSCs-EXO: paracrine mechanisms, immunomodulatory and anti-inflammatory effects, as well as their promotion of tissue regeneration. This review also examines the role of MSCs and MSCs-EXO in cardiovascular diseases, neurological disorders, autoimmune diseases, musculoskeletal disorders, and other systemic diseases over the past five years, while discussing the challenges and difficulties associated with their clinical application. Finally, we systematically summarized and analyzed the potential causes of the various heterogeneous results currently observed. Additionally, we provided an in-depth discussion on the challenges and opportunities associated with the clinical translation of disease treatment approaches based on MSCs, MSCs-EXO, and engineered exosomes.
Similar content being viewed by others
New therapeutic approaches of mesenchymal stem cells-derived exosomes
Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials
Translational potential of mesenchymal stem cells in regenerative therapies for human diseases: challenges and opportunities
Explore related subjects
Discover the latest articles, books and news in related subjects, suggested using machine learning.
Introduction
The concept of stem cell was first proposed in 1868 to describe the properties of fertilized egg [1]. It was identified in murine bone marrow and characterized based on their multilineage differentiation potential [2]. The term of mesenchymal stem cells (MSCs) was formally introduced and separated in 1991 as a distinct category of stem cells, and has been widely utilized in research and clinical applications since then [3]. MSCs are pluripotent stem cells with the capacity of proliferative and self-renewal, homing, pluripotency and low immunogenicity. MSCs come from a wide range of sources. In addition to bone marrow, they can usually be isolated in adipose tissue, dental pulp, umbilical cord and placenta [4].
In 1967, extracellular vesicles (EVs) secreted by chondrocytes was first observed by E Bonucci [5]. EVs are a class of lipid bilayer particles secreted by cells, which are primarily composed of exosomes (50–150 nm) and micro-vesicles (50–1000 nm) [6]. Exosomes, a subtype of EVs, exhibit a topological structure similar to that of cells [7]. In the 1980s, it was established that the normal synthesis and secretion of exosomes are essential for certain physiological processes [8]. Furthermore, subsequent research has indicated that exosome secretion may play a role in the quality control of specific plasma membrane proteins [9]. An increasing body of research has demonstrated that exosomes can mediate intercellular communication via receptor-ligand interactions, participate in a wide range of cellular processes, and play a significant role in the pathogenesis and progression of various diseases [10,11,12].
In recent years, MSCs and the MSCs-derived exosomes (MSCs-EXO) have demonstrated significant research advances in the treatment of a variety of diseases, such as cardiovascular diseases (CVDs), neurological disorders, autoimmune diseases (ADs), musculoskeletal disorders (MSDs) and some other diseases. This review will focus on the properties and features of different types of MSCs and MSCs-EXO. In addition, we described the mechanism of MSCs and MSCs-EXO in treating diseases. Moreover, we summarize the recent application of MSCs and MSCs-EXO in the clinical treatment of the aforementioned diseases. Finally, we discuss the current status, limitations and future challenges for the application of MSCs and MSCs-EXO in clinical.
Classification and characterization of MSCs
MSCs, a type of multipotent stromal cells, can adhere to the bottom of the petri dish in cell culture. It can express surface molecules such as CD105, CD73 and CD90, and hardly expresses CD45, CD34, CD14 or CD11b, CD79α or CD19+, HLA-DR+ [13]. Initially, MSCs were predominantly isolated from bone marrow for applications in disease treatment and tissue repair. However, due to the process of bone marrow-derived MSCs (BM-MSCs) is invasive and painful, researchers have gradually shifted the focus to isolating MSCs from other alternative tissues such as adipose tissue, umbilical cord, and dental pulp. According to their sources, MSCs can be classified into the following categories: BM-MSCs, adipose tissue-derived MSCs (AD-MSCs), umbilical cord-derived MSCs (UC-MSCs), placenta-derived MSCs (P-MSCs), dental pulp-derived MSCs (DP-MSCs), and MSCs from other sources [14]. This section will provide a comprehensive overview of the sources, properties, and characteristics of the above common cell types of MSCs.
Bone marrow-derived MSCs
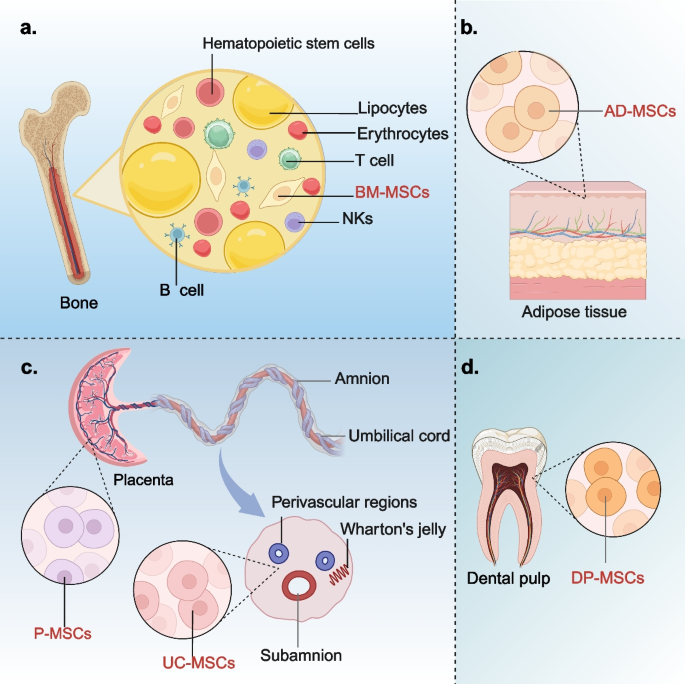
In 1976, Friedenstein et al. [15] initially discovered MSCs in the bone marrow of adult mice and named them fibroblasts precursor cells for the first time, also known as BM-MSCs (Fig. 1a). Although the content of BM-MSCs in bone marrow is very small, BM-MSCs are widely used in the treatment of multiple diseases due to their capacity of rapid proliferation, highly stable genetic characteristics, low immunogenicity and excellent multiline differentiation potential [16,17,18]. The cellular properties of BM-MSCs are usually closely related to the physical health status, pathophysiological status and the age of donor. For example, as donor age increases, indicators of aging in BM-MSCs also rise, which include oxidative damage, the elevated levels of reactive oxygen species (ROS), and the increased expression of p21 and p53 [19]. Consequently, BM-MSCs exhibit enhanced differentiation tendencies. This means that the adaptability and therapeutic effectiveness of BM-MSCs will decline with the increasing of donor ages.
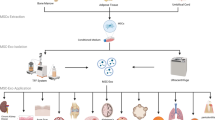
Overview of the classification and characteristics of MSCs. Different types of MSCs are derived from different tissues, mainly from bone marrow (a), adipose tissues (b), umbilical cord and placenta (c), and dental pulp (d). MSCs: mesenchymal stem cells; MSCs-EXO: MSCs-derived exosomes; BM-MSCs: bone marrow-derived MSCs; AD-MSCs: adipose tissue-derived MSCs; UC-MSCs: umbilical cord-derived MSCs; P-MSCs: placenta-derived MSCs; DP-MSCs: dental pulp-derived MSCs
Bone marrow is a complex tissue comprising various cell types. In addition to BM-MSCs, it also contains non-MSCs populations such as hematopoietic stem cells, red blood cells, and various immune-related cells. These diverse cell types collaborate to support hematopoiesis and the immune system. Traditional methods for the isolation and culture of BM-MSCs primarily rely on purification based on intrinsic cellular characteristics, including adhesion time, growth patterns, and tolerance to digestive enzymes during passaging. However, BM-MSCs are not the dominant cell population in the bone marrow. It is hard to develop BM-MSCs into the dominant cell population in the process of isolation and culture. Furthermore, BM-MSCs are frequently co-isolated with other cell types, which poses significant challenges for their purification and subsequent clinical application. In recent years, with the continuous innovation of biological technology, new methods such as density gradient centrifugation, hyaluronic acid hydrogel and flow cytometry have greatly improved the isolation purity of BM-MSCs [20]. These new methods provide a guarantee for further study of the function and clinical application of BM-MSCs. Among the standards established by the International Society for Cell Therapy (ISCT), the three minimal criteria for identifying BM-MSCs are as follows: First, cells must exhibit adherence to plastic substrates. Second, cells must express specific surface antigens, including positivity for CD105, CD73, and CD90, and negativity for CD45, CD34, CD14 or CD11b, and CD79a or CD19. Third, under standardized in vitro culture conditions, these cells must demonstrate the potential to differentiate into osteoblasts, adipocytes, or chondrocytes [21].
It is worth mentioning that the separation method of density gradient centrifugation is usually applicable to the separation of liquid cells and can accurately separate BM-MSCs separated from bone marrow cell suspensions. However, it cannot be applied to tissue matrices characterized by solids such as fat or umbilical cord and placenta [22]. Furthermore, compared with the density gradient centrifugation method, directly isolating BM-MSCs from bone marrow by leveraging their characteristic adhesion to culture plastic is a more advantageous approach. This method can significantly enhance the expression level of HLA-DR, which may serve as a potential marker for evaluating the quality control of MSCs in certain contexts [23]. In addition, during the amplification of BM-MSCs, cell density serves as a critical parameter. Specifically, when the cell density in the ranges of T25 culture flask between 10,000 and 100,000 cells/cm2, the expansion rate of MSCs can be significantly enhanced, thereby facilitating large-scale cell culture [24]. After being cultured in vitro for 5 to 7 days, plastic-adherent MSCs may exhibit heterogeneous morphologies resembling fibroblasts, including endothelial cells (ECs), under microscopic observation. During the amplification process, the heterogeneity of adherent MSCs gradually diminishes, with fibroblast-like cells progressively becoming the predominant cell population [21]. The common traditional conditioned medium used for the in vitro culture of BM-MSCs mainly include DMEM/F12, α-MEM and DMEM which supplemented with 10%−15% fetal bovine serum (FBS) [25,26,27]. However, it has been shown that traditional conditioned medium of BM-MSCs can repair damaged tissues and has the characteristics of high immune compatibility, there are still some safety problems such as allergy in clinical application. In this context, FBS is frequently employed as a supplementary component in traditional culture medium for MSCs. But its use may introduce risks of immunogenicity or contamination. In contrast, human platelet lysate (HPL) has been demonstrated to be a safe and efficient alternative to FBS. HPL effectively mitigates the risks of immune reactions and infections associated with FBS, supports robust MSCs expansion, and holds promise as a viable substitute for FBS in MSCs culture [24]. In recent years, the traditional conditioned medium has been progressively supplanted by emerging serum-free conditioned medium. Compared with the traditional conditioned medium, serum-free conditioned medium can better expand BM-MSCs in vitro, maintain the pluripotency of BM-MSCs, and shorten the culture cycle [28]. This provides a safe guarantee for the clinical application of BM-MSCs.
Adipose tissue-derived MSCs
AD-MSCs are pluripotent stem cells derived from adipose tissues with the potential to differentiate into various cell lineages (Fig. 1b). More than 90% of the surface antigens on the cell membrane of AD-MSCs are identical with BM-MSCs [29, 30]. Compared to BM-MSCs, AD-MSCs have a broader range of tissue sources. Additionally, the yield of MSCs from adipose tissue is significantly higher than that from bone marrow, and the quantity of AD-MSCs obtained in a single extraction is sufficient to meet the requirements of clinical applications [31]. It can be speculated that the use of freshly isolated cells is likely to be safer compared to cells subjected to in vitro culture and secondary expansion. This is due to the fact that during prolonged in vitro culture, cumulative genetic and epigenetic alterations may occur, potentially affecting cellular function and biological properties [32]. BM-MSCs extraction from bone marrow is a painful process, which often puts stress on the donor. However, the extraction of AD-MSCs from adipose tissue is less psychologically stressful for the donor and more acceptable. Therefore, this also increases the motivation for donors to donate AD-MSCs. Compared with BM-MSCs, AD-MSCs have more stable morphological and genetic characteristics, higher proliferative activity and lower senescence rate under the same in vitro culture conditions [33, 34]. Unlike the isolation of BM-MSCs, the isolation of AD-MSCs necessitates the use of digestive enzymes to separate AD-MSCs from the surrounding fascia, adipocytes, and vascular ECs [35]. Consequently, the process of isolating AD-MSCs demands precise control over the duration of enzymatic digestion to prevent significant adverse effects on cell viability and reparative capacity.
Umbilical cord-derived and placenta-derived MSCs
In the 1990 s, UC-MSCs were first isolated from connective tissue [36]. The current study shows that UC-MSCs are isolated from four main sites in the umbilical cord: Wharton’s jelly, perivascular regions, subamnion and amnion. Wharton’s jelly region is the main source regions of UC-MSCs (Fig. 1c) [37]. Li et al. compared MSCs from four different sources and found that UC-MSCs from Wharton’s jelly proliferate the fastest which are easy to be isolated and culture, and can maintain the characteristics through multiple generations in vitro [38]. In addition, UC-MSCs express almost no MHC II antigens associated with homoimmune rejection and have undergone barely any cellular ageing [39]. Therefore, compared with adult MSCs, UC-MSCs exhibit lower immunogenicity, non-tumorigenicity, decreased heterogeneity, and enhanced pluripotency. [40]. This means that UC-MSCs have better clinical application potential than adult MSCs. In vitro, The isolation and culture of UC-MSCs can be obtained not only by enzymolysis of collagenase, hyaluronidase or other proteases, but also by explants. UC-MSCs are easy to proliferate and culture, and no ethical concerns about their collection [41, 42]. Compared with UC-MSCs obtained by enzymatic hydrolysis, the number of UC-MSCs obtained from explants was higher due to the significant up-regulation of mitosis and cell cycle related gene expression in acquired cells [43]. These results indicate that enzymatic hydrolysis technology has certain limitations due to its ability to destroy cell surface structures and proteins and reduce cell viability. Explants culture is a milder technique for the isolation and culture of UC-MSCs than enzymatic hydrolysis.
Consistent with the umbilical cord, the placenta also originates from mesenchymal tissue during embryonic development and shares a similar developmental biological background [44]. Therefore, whether it is UC-MSCs or P-MSCs (Fig. 1c), they all have similar immunomodulatory characteristics, low immunogenicity, and consistency in cell morphology [45]. However, owing to the complexity of the placental tissue structure, this typically entails that when preparing P-MSCs, more sophisticated isolation and purification procedures are required to achieve P-MSCs with higher purity [46].
Dental pulp-derived MSCs
In recent years, DP-MSCs, as an alternative cell source, have demonstrated significant therapeutic potential and have garnered increasing attention from researchers. DP-MSCs are a unique pluripotent cell population with significant mesenchymal characteristics [47]. Different from the origin of BM-MSCs and AD-MSCs, DP-MSCs are mainly derived from the embryonic layer of neural crest and have certain neurophilic characteristics (Fig. 1d) [48]. It is easily isolated and obtained from human third molars removed for orthodontic reasons, and can also be isolated and extracted from the pulp of animals (including dogs) [49]. The abundant cell supply and painless stem cells collection method of DP-MSCs make them a rich source of cells for regenerative medicine with less risk of complications. In addition, DP-MSCs also have advantages such as high proliferation capacity, good differentiation potential, favorable paracrine, minimal invasion, and immune regulation [50]. As a mesenchymal cell population in dental pulp, DP-MSCs have stem cells’ properties expressed by specific markers [51]. In a preclinical study, DP-MSCs were found to express embryonic stem cell markers such as SKIL, MEIS1, and JARID [52]. This suggested that DP-MSCs have the same immunomodulatory and regenerative characteristics as embryonic stem cells. However, there are still other cells in the pulp, such as fibroblasts, highly differentiated dentin-forming odontoblasts, mesenchymal progenitor cells, and perivascular cells. When isolating DP-MSCs, surface markers for positive characterization of MSCs (primarily CD73, CD90, and CD105) are commonly shared with these cells [21]. The detection of a single marker is insufficient to determine the purity of DP-MSCs, and multiparameter flow cytometry addresses this limitation by simultaneously detecting multiple molecular markers [51]. Colony separation is a cell isolation technique that distinguishes the proliferative and self-renewal capacities of stem cells based on cell surface markers, as well as the size and morphology of colonies formed during cell culture. This method is primarily used to isolate DP-MSCs with distinct mesenchymal surface markers; however, it is not suitable for obtaining homogeneous populations of DP-MSCs [53]. Homogeneous populations of DP-MSCs may express similar or identical surface markers, and may have a high degree of morphological and functional consistency during cell culture [54]. When cultured under 3D conditions that more closely mimic the in vivo environment, DP-MSCs can better preserve their original properties, in contrast to the conventional 2D culture environment typically used in standard cell culture dishes [55]. The difference of cell culture density has different effects on the maintenance of stem cell characteristics. The confluent culture condition had a negative effect on the maintenance of stem cell characteristics, while the sparse culture condition had the opposite effect [56]. However, in the densely cultured DP-MSCs, the expression of PI3 K was upregulated [55]. PI3 K catalyzes the intracellular conversion of PIP2 to PIP3. PIP3 recruits AKT to the cell membrane and promotes its activation. Activated AKT can affect the differentiation direction of DP-MSCs and promote their proliferation [57]. Perhaps when DP-MSCs are used for some tissue regeneration, confluent culture may be an alternative strategy.
Other sources
In recent years, researchers have gradually discovered that MSCs can be successfully isolated from other tissues such as the endometrium [58], peripheral blood [59], ectodermal tissues (such as keratin of the cornea, [60] skin and accessory organs of the eye [61]), skeletal muscle and muscle [59, 62], venous vessels [62], peripheral blood [63] and the fetus including the heart, intestinal epithelium and dermal tissue [64]. However, these tissues do not have a stable source. Ethically, it is also not suitable as a tissue source for large-scale clinical production of MSCs.
Exosomes and MSCs-derived exosomes
Characterization and isolation of exosomes
Exosomes are small membrane-bound vesicles of endocytic origin, which can be produced by nearly all organisms and cell types [65]. Specific proteins, lipids, nucleic acids, and glycoconjugates formed the structure of exosomes [66]. In less than five decades since their discovery, exosomes have emerged as a significant research focus due to their advantages such as high safety, easy preservation and transportation, no ethical controversy, and obvious therapeutic effect. Currently, the extraction methods of exosomes mainly include differential ultracentrifugation, ultrafiltration, size-exclusion chromatography, polymer precipitation, microfluidics and immunoaffinity capture. Among these, the gradient ultrafast centrifugation is the most common extraction method [67]. This technique involves the sequential application of increasing centrifugal forces to separate exosomes from other cellular components based on their buoyant densities. The process typically involves multiple rounds of centrifugation, yielding purified exosomes in the final pellet [68]. However, this method comes with several drawbacks, including the high cost of equipment, the time-consuming nature of the process, and the instability in the number of exosomes obtained. In recent years, the method of polymerization-induced precipitation has gained considerable popularity among researchers due to its cost-effectiveness and high yield, and has emerged as a viable alternative, gradually becoming the second choice for exosome extraction, as evident in various studies [69]. The principle of polymerization-induced precipitation is similar to Ethanol-mediated nucleic acid precipitation which forms a hydrophobic microenvironment through the interaction of highly hydrophilic polymers with water molecules around exosomes. This causes the exosomes to precipitate [70]. However, compared with differential ultracentrifugation, this method can lead to non-specific protein contamination and may influence the analysis of downstream results [71]. For exosomes that have been subjected to extraction and purification, a comprehensive quality assessment is typically performed from the perspectives of particle size distribution, quantification, and biological activity. When performing quality assessment, a range of advanced techniques can be employed, such as nanoparticle tracking analysis (NTA) [72], transmission electron microscopy (TEM) [73], atomic force microscopy (AFM) [74], and Western blotting [74]. These techniques not only enable precise evaluation of the size distribution, morphological characteristics, purity level, and protein composition of exosomes but also ensure that the extracted exosomes comply with the anticipated quality and purity standards. Moreover, exosomes demonstrate remarkable resilience to various storage conditions. They can be preserved under freezing conditions or via freeze-drying technology, which substantially decreases costs associated with manufacturing, storage, and transportation [74]. Therefore, selecting an appropriate exosome extraction method, as well as quality control and storage approaches, is of particular importance for ensuring the accuracy of subsequent experimental results.
Roles of exosomes in cell communication
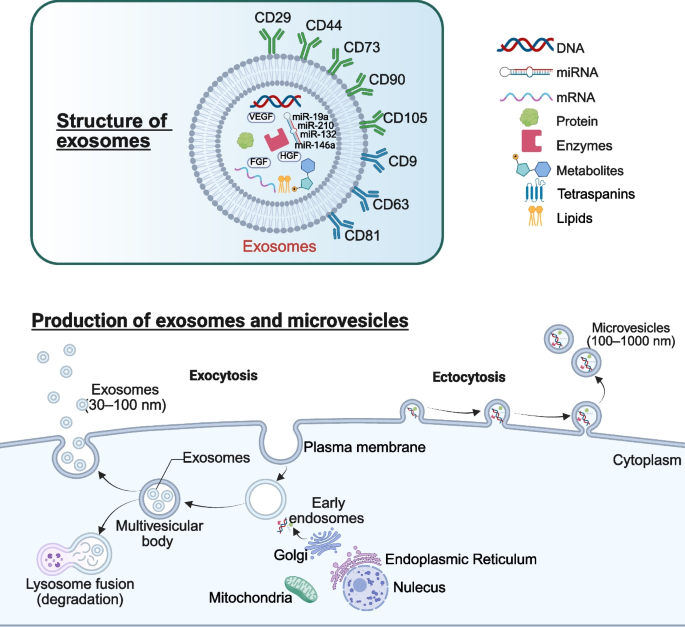
The production process of exosomes primarily involves several key steps: the formation of the plasma membrane, the sorting of endosomes and multivesicular bodies, and the release of exosomes through the fusion of multivesicular bodies with the plasma membrane [75]. During the biogenesis of exosomes, various substances, including nucleic acids (miRNA, long non-coding RNA, circRNA, and DNA), proteins, lipids, and even mitochondria, are encapsulated and subsequently delivered to target cells [76]. MiRNAs, as one of the key molecular cargos delivered by exosomes, are initially transcribed into primary miRNAs in the nucleus. These primary miRNAs are subsequently processed by the Drosha enzyme to generate precursor miRNAs, which are then transported to the cytoplasm via Exportin-5. In the cytoplasm, these precursors are further processed into mature miRNAs. During exosome biogenesis, mature miRNAs can associate with RNA-induced silencing complexes (RISCs) and subsequently be encapsulated within exosomes, thereby becoming functional components of these EVs [77]. MiRNAs not only protect exosomes from enzymatic degradation during delivery but also facilitate their transfer to target cells via endocytosis or by binding to specific receptors. Additionally, miRNAs can bind to the 3’UTR region of target-cell mRNA, thereby inhibiting translation or inducing degradation and consequently modulating the expression of downstream genes [78].
Mitochondria, as important organelles within cells, can regulate energy metabolism, apoptosis and cell signal transduction. The transcellular transfer of mitochondria is the key mechanism to ensure the smooth execution of its functions. Exosomes, as one of the microtubule cytoskeletons with common intracellular movement patterns, can provide significant impetus for the transport complexes on the cytoskeleton to drive mitochondrial movement [79]. Under the stimulation of energy stimulation, oxidative stress or DNA damage, the intracellular ROS level increases, and exosomes can achieve mitochondrial transcellular transfer. Research has found that astrocytes can release a large number of exosomes containing mitochondria under the conditions of stimulating ischemic stroke in vivo and excreting serum in vitro, reducing peripheral nerve damage [80]. In a mouse model of lipopolysaccharide-induced lung injury, exosome-mediated functional mitochondrial transfer can promote anti-inflammatory and antioxidant stress in mouse macrophages [81]. As a transmembrane protein, the NAD +/CD38/cADPR/Ca2+ pathway mediated by CD38 is the key to exosome-mediated mitochondrial transcellular transport. This pathway can not only be involved in the endocytosis stage of exosomes, but also in the exocytosis stage, influencing intercellular communication and material exchange [82]. The inhibition of endocytosis will lead to a reduction in mitochondrial transfer from BM-MSCs to damaged alveolar epithelial cells. Furthermore, Connexin 43 (CX43), as a connexin of mitochondria, the interstitial junction channels it mediates can significantly enhance the efficiency of mitochondrial transcellular transfer. Under certain conditions, it can protect myocardial and brain tissues from ischemia–reperfusion injury and is involved in the endocytosis of reticin-dependent exosomes [83].
Exosomes are secreted from parent cells and their targeting mechanisms remain incompletely understood. Research has demonstrated that exosomes can be internalized by recipient cells through protein–protein interactions (such as fusion with the plasma membrane and direct interaction with surface receptors) or receptor-ligand interactions (including caveolae-dependent or caveolae-independent endocytosis), thereby performing their functional roles [84]. In the first way, integrins, tetraspanins, and other adhesion proteins play a crucial role in exosome targeting and the selective uptake by recipient cells [85]. Integrins are cell adhesion molecules that play an indispensable role in regulating cell growth and function. Targeting integrins α6β4 and αvβ5 can reduce the uptake of exosomes by lung-tropic and liver-tropic tumor cells, thereby decreasing metastasis to the lung and liver, respectively [86]. Connexins are another class of tetraspanins that facilitate intercellular communication on exosome membranes, in addition to integrins. Connexins are primarily composed of two extracellular loops and one cytoplasmic loop, enabling their biological functions through connexin-integrin cross-talk. Notably, CX43, a key member of the connexin family, plays a significant role in regulating exosome release and molecular transfer [87, 88]. CD81, CD9, and CD63 were initially identified as tetraspanin markers of exosomes. However, subsequent research revealed that only CD63 is a specific protein marker for exosomes, whereas CD9 and CD81 are not [89]. Moreover, recent studies have shown that in certain cell types, the absence of these three proteins has minimal impact on the protein composition of exosomes [90]. Previous studies have demonstrated that inhibiting endocytosis in recipient cells can reduce their uptake of exosomes. However, the relationship between endocytosis and exosome release within the same cell remains undetermined. Recent research indicates that endocytosis can inhibit the secretion of vesicular exosome marker proteins (CD81, CD9, and CD63). Additionally, endocytosis can trigger the degradation of exosomes containing CD9 and CD81 [91]. This suggests that cells can either primarily release exosomes as parent cells or simultaneously receive exosomes. This finding provides valuable insights for future research on the production and modification of post-therapeutic exosomes. Exosomes, as secretory vesicles, can be utilized by adjacent cells to exert their functions. For instance, after entering the bloodstream, exosomes can be transported over long distances to reach lesion sites and deliver therapeutic effects. However, hepatic accumulation of exosomes can diminish their therapeutic efficacy [92]. Therefore, engineering exosomes holds significant potential for enhancing their therapeutic performance. Currently, researchers have demonstrated that blocking Scavenger Receptor Class A (a novel monocyte/macrophage uptake receptor for EVs) with dextran sulfate significantly reduces the clearance of EVs in the liver [93]. This finding is crucial for enhancing the effective utilization of exosomes.
MSCs-derived exosomes
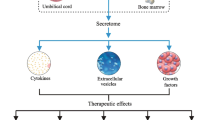
MSCs can secrete a lot of secretomes, including cytokines, growth factors and chemokines, and extracellular nanoparticles dominated by exosomes [48]. Compared to the challenges of iatrogenic tumorigenesis, immune rejection, vascular blockage, and ethical concerns faced by MSCs in clinical applications, MSCs-EXO not only effectively mitigate these issues but also exhibit comparable reparative effects to those of MSCs [94]. MSCs-EXO carry not only the characteristic markers of MSCs (such as CD29, CD44, CD73, CD90, and CD105), but also the characteristic markers of exosomes (such as CD9, CD63, and CD81) [95]. MSCs-EXO primarily exerts its functions by transferring various bioactive molecules, including growth factors (such as VEGF, FGF, HGF), anti-inflammatory proteins, anti-apoptotic proteins, cell survival-promoting factors, angiogenic proteins, microRNAs (such as miR-19a, miR-132, miR-146a, miR-210), long non-coding RNAs, circRNAs, mRNAs, and lipids (Fig. 2) [76, 96,97,98,99,100,101,102,103,104]. This is usually achieved by the target cell internalizing MSCs-EXO into the cell through endocytosis. After the proteomics analysis of AD-MSCs-derived exosomes (AD-MSCs-EXO), BM-MSCs-derived exosomes (BM-MSCs-EXO), UC-MSCs-derived exosomes (UC-MSCs-EXO) and DP-MSCs-derived exosomes (DP-MSCs-EXO). Zheng-Gang Wang et al. found 771, 457 and 431 proteins respectively, 355 of which were common proteins [105]. This study indirectly suggests that different MSCs-EXO may have different therapeutic effects on the same disease. In addition, miRNA is also an important component of MSCs-EXO and plays an important role in the treatment of various diseases [106,107,108]. The types and amounts of proteins and miRNAs in MSCs change with the aging of MSCs [109, 110]. Therefore, it can be speculated that the contents of MSCs-EXO will also change with the aging of MSCs, and the cell viability of MSCs will directly affect the therapeutic effect of MSCs-EXO.
Overview of the characteristics and formation process of exosomes. The formation process of exosomes mainly includes: the formation of plasma membrane, the sorting endosomes and multivesicular body and the release of exosomes by the combination of multivesicular body and plasma membrane
Mechanisms Underlying the Therapeutic Effects of MSCs and MSCs-EXO
Paracrine Signaling
Paracrine pathway is considered to be the primary pathway for stem cell therapy. In a narrow sense, paracrine factors with diameters less than 10 nm secreted by stem cells are referred to as paracrine substances, and the term paracrine encompasses all secretory products, including exosomes and other cellular vesicles in a broader context [111]. Extensive research has demonstrated that stem cells secrete a wide array of bioactive molecules, such as anti-inflammatory factors, growth factors, cytokines, and specific active factors [112, 113]. These secretomes play crucial roles in regulating stem cell homing, enhancing self-survival, promoting anti-apoptotic effects, stimulating angiogenesis, modulating immune responses, inhibiting excessive fibrosis, and facilitating cell proliferation, survival, and differentiation [114, 115].
The conditioned medium from stem cells demonstrates a therapeutic efficacy comparable to that of stem cells themselves, providing robust evidence that the paracrine mechanism plays a crucial role in the disease-treating effects of stem cells. For instance, the conditioned medium from DP-MSCs not only alleviates cardiomyocyte apoptosis in ischemia–reperfusion (I/R) mouse models but also inhibits I/R-induced cardiomyocyte apoptosis in vitro [116]. For AD-MSCs, their conditioned medium could attenuate I/R-induced cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway [117]. In addition, conditioned medium from AD-MSCs could also protect neuronal and oligodendroglia cells exposed to oxygen–glucose deprivation (OGD) [118]. It is well established that external stimuli enhance the paracrine activity of MSCs, offering valuable insights for leveraging this property in disease treatment. Therefore, hypoxia [119], ischemia [120], carbon monoxide exposure [121], physical factors [122], beneficial chemical elements [123], cytokines [124], and pharmacological agents [125] can be employed to enhance the paracrine effect and efficacy of MSCs. For example, under ischemic conditions, MSCs can mitigate cardiomyocyte apoptosis by secreting exosomes enriched with miR-22 [126, 127]. Compared with UC-MSCs in normal oxygen environment, hypoxia-preconditioned UC-MSCs have better angiogenesis in the treatment of myocardial infarction (MI) mice. Furthermore, the latter conditioned medium can better promote the formation of human umbilical vein EC tubules in vitro [128]. In the osteochondral defect model, hypoxia-preconditioned BM-MSCs facilitate cartilage regeneration and attenuate joint inflammation through the mediation of EVs [129]. MSCs pretreated with metformin can enhance their proliferative capacity and activity by augmenting their paracrine effects, thereby mitigating inflammation and fibrosis in chronic kidney disease (CKD) models [130].
The combination of MSCs with gene editing technology, or the integration of MSCs with other therapeutic modalities, represents another important strategy for enhancing paracrine activity. For example, the genes associated with hypoxia resistance and enhanced migration, including HIF-1α [131], SDF-1 [132], HO-1 [133], CXCR4 [134], and specific miRNAs [135,136,137], were transferred into MSCs through gene editing technology could improve the repair ability of MSCs. The combination of pharmacological agents, physical factors, and novel polymeric materials with MSCs can improve therapeutic efficacy and augment immune modulation [138,139,140,141,142]. Although the synergistic mechanisms underlying combination therapy have not been fully elucidated, several studies suggest that they may be associated with enhanced paracrine effects of MSCs [143, 144]. Therefore, actively elucidating the mechanisms by which combination therapy achieves a synergistic effect on paracrine signaling will be a key focus of future research.
Immunomodulatory and anti-inflammatory
Innate immunity is an important part of immune response. Cells involved in innate immunity primarily include neutrophils, monocytes, macrophages, and dendritic cells (DCs), as well as specific lymphocyte populations such as natural killer (NK) cells and γδ T cells. These cells secrete inflammatory mediators like ROS, IL-6, and TNF-α in response to various exogenous and endogenous stimuli, thereby inducing an inflammatory response [145].
Within the first few hours following tissue damage, innate immune responses will gradually be initiated. During this process, in addition to growth factors, chemokines, and pro-fibrotic factors, the damaged tissues can also release a large number of inflammatory factors. The change of microenvironment and the accumulation of inflammatory factors can lead to the increase of vascular permeability and drive inflammatory cells to gather at the damaged area [146]. Various pro-inflammatory cytokines and chemokines released by activated macrophages co-create a pro-inflammatory environment with cytokines released by damaged tissue. This pro-inflammatory environment attracts neutrophils to the damaged area to participate in early inflammatory responses. This process usually occurs within 6 h after tissue damage [147]. Adaptive immunity, primarily mediated by B and T lymphocytes, enables the recognition of specific pathogens and the generation of pathogen-specific antibodies. For example, in the treatment of malignant tumor diseases, MSCs can stimulate central granulocytes to secrete IL-10 and PGE2, thereby inhibiting the proliferation of T lymphocytes in the tumor microenvironment [148]. This immune response plays a crucial regulatory role in the pathogenesis and progression of diseases such as atherosclerosis, systemic lupus erythematosus (SLE), cancer and osteoarthritis (OA) [148,149,150,151,152].
The immune response can both inflict damage on tissues and trigger the wound healing process, including the formation of scar tissue [153]. Therefore, there exists a delicate balance between the damaging and reparative effects during the inflammatory response. If this balance is disrupted, it can lead to either an excessive inflammatory response or inadequate repair of tissue damage. Consequently, maintaining the equilibrium between pro-inflammatory and anti-inflammatory responses is essential for optimizing the protective effect on injured cells. For example, on the first day after myocardial damage, the activated M1 macrophages and their secreted cytokines, together with attracted neutrophils, create a more intense pro-inflammatory environment. More immune cells are attracted and additional damage is caused to cardiomyocytes [147]. However, macrophages can also change from M1 type to M2 type, and the cytokines secreted by M2 type macrophages are mainly anti-inflammatory, wound healing and angiogenesis [154]. This is crucial for the protection and preservation of damaged cardiomyocytes. In recent years, a substantial body of research has elucidated the significant role of MSCs and MSCs-EXO in immunomodulatory and anti-inflammatory across various diseases, including MSDs, CVDs, neurological disorders, ADs, and others.
OA is a representative disease of MSDs. It is a complex, multifactorial degenerative condition that arises from the interplay of biomechanical factors and inflammatory processes. MSCs and MSCs-EXO can attenuate the activity of pro-inflammatory immune cells and modulate local and systemic biomarkers, thereby regulating the immune response in OA (Fig. 3) [155]. Mitochondria are key organelles in oxidative stress. Mitochondria derived from MSCs can reverse abnormalities in energy metabolism and mitochondrial dynamics in chondrocytes in OA, thereby enhancing chondrocytes’resistance to oxidative stress and apoptosis [156]. In addition, MSCs-EXO can alleviate cartilage degradation and synovial inflammation in OA through ferroptosis [157]. Furthermore, the synergistic integration of biological scaffolds composed of polymer materials with MSCs can augment their anti-inflammatory effects in the OA microenvironment [158, 159].
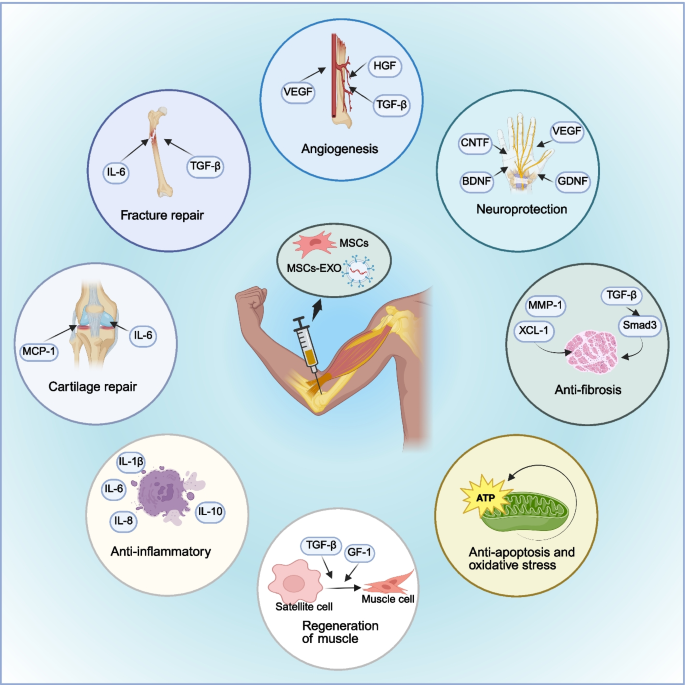
Application of MSCs and MSCs-EXO in MSDs. MSCs and MSCs-EXO are primarily administered via intra-articular injection to the affected site, exerting therapeutic effects such as fracture repair, cartilage regeneration, anti-inflammatory responses, muscle regeneration, anti-apoptosis, oxidative stress reduction, anti-fibrosis, neuroprotection, and angiogenesis for the treatment of musculoskeletal diseases. MSCs: mesenchymal stem cells; MSCs-EXO: MSCs-derived exosomes; MSDs: musculoskeletal disorders
Neurological disorders are frequently associated with neuroinflammation (Fig. 4). Neuroinflammation is activated by damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs) [160]. Upon damage to neurons and glial cells, the DAMPs secreted by these cells can activate microglia and astrocytes, leading to changes in their morphology and function [161]. Microglia and astrocytes serve as the primary effector cells of the innate immune response in the central nervous system. Their activation subsequently enables these cells to exert their immunomodulatory effects. Similar to macrophages, activated microglia exhibit two distinct phenotypes: M1 and M2. The M1 phenotype is primarily associated with pro-inflammatory responses, whereas the M2 phenotype is predominantly involved in anti-inflammatory and neuroprotective functions [162]. Astrocytes can also produce pro-inflammatory or immunomodulatory mediators depending on their polarization phenotype [163]. MSCs represent an ideal cell source for neural reparation and inflammation reduction due to their low immunogenicity and paracrine effects. MSCs primarily modulate neuroinflammation through their interactions with microglia and astrocytes. For example, MSCs-EXO can inhibit inflammation and promote the recovery of neurological function by entering microglia and suppressing their activation [164]. Furthermore, MSCs-EXO enriched in miR-216a-5p can also regulate microglial polarization by modulating the TLR4/NF-κB/PI3 K/AKT signaling pathway [165]. GFAP and IBA1 serve as markers for astrocytes and microglia, respectively. Exosome therapy can significantly reduce the transcription levels of inflammatory markers in astrocytes and microglia, indicating a diminished inflammatory state following spinal cord injury (SCI) [166]. Post-stroke inflammatory injury is a critical factor that exacerbates stroke-induced brain damage. MSCs overexpressing HO-1 exhibit a significant therapeutic effect on hyperinflammatory injury following stroke in mice, ultimately promoting recovery after ischemic stroke [167].
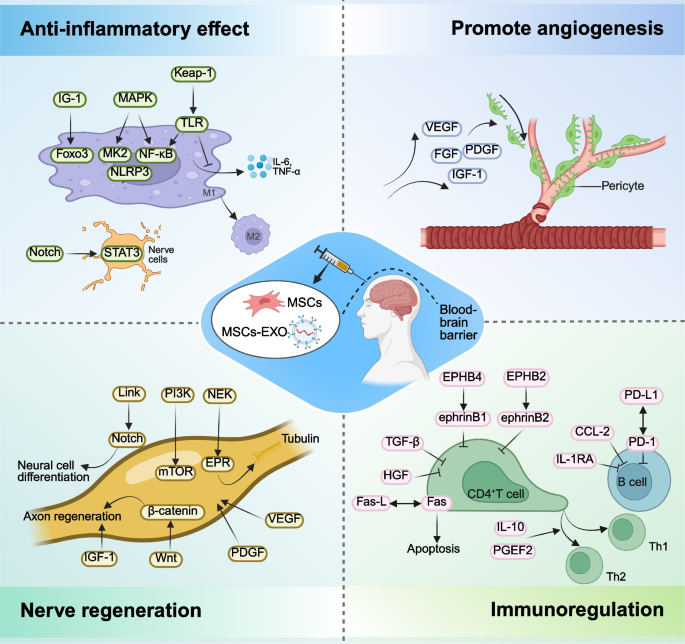
Application of MSCs and MSCs-EXO in neurological disorders. MSCs and MSCs-EXO can exert therapeutic effects by reducing inflammation, promoting angiogenesis, facilitating nerve regeneration, and modulating immune responses. MSCs: mesenchymal stem cells; MSCs-EXO: MSCs-derived exosomes
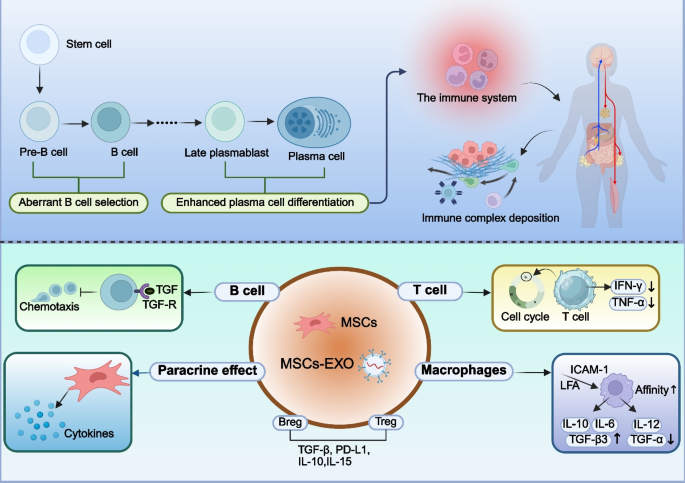
ADs comprise a group of chronic inflammatory disorders characterized by the immune system attacking the body’s own tissues, leading to the formation and deposition of immune complexes [168]. MSCs and MSCs-EXO can elicit either inhibitory or stimulatory immune responses, thereby participating in immune regulation and modulation. The aberrant expression of autoantibodies is a key mechanism underlying the occurrence and development of AD, and the emergence of these autoantibodies is frequently attributed to aberrant B cell selection or enhanced plasma cell differentiation, indicating a breakdown in self-tolerance (Fig. 5) [169]. MSCs and MSCs-EXO contribute to the treatment of AD by modulating the aberrant expression of autoantibodies [170]. BM-MSCs can inhibit antigen-dependent proliferation and differentiation into plasma cells of follicular and marginal zone B cells in SLE mice via the IFN-γ and PD-1/PD-L1 pathways, thereby contributing to the prevention of glomerular damage [171].
Application of MSCs and MSCs-EXO in ADs. ADs primarily arise from aberrant B cell selection and enhanced plasma cell differentiation, leading to the immune system attacking normal human tissues. This results in the deposition of immune complexes. MSCs can inhibit the proliferation of B cells, T cells, and macrophages, promote the release of anti-inflammatory factors, and reduce the production of pro-inflammatory factors. Furthermore, MSCs facilitate the differentiation of T cells and B cells into regulatory subsets, such as Tregs and Bregs. ADs: autoimmune disease; MSCs: mesenchymal stem cells; MSCs-EXO: MSCs-derived exosomes; Treg: regulatory T cells; Breg: regulatory B cells
CD4 + T cells also play a crucial role in the pathogenesis of multiple AD, contributing to target organ damage through the production of cytokines such as IFN-γ, TNF-α, IL-4, IL-17, IL-21, and IL-23 [171, 172]. For example, MSCs can promote the expression of IL-10, downregulate the expression of IL-18 and Th17 cells, thereby correcting the Th17/Treg (a type of CD4 + T) imbalance to exert immunomodulatory effects [173]. Currently, glucocorticoids serve as the cornerstone therapy for the treatment of most AD. Dexamethasone-integrated MSCs and MSCs-EXO can treat SLE by inhibiting the release of IFN-γ and TNF-α from CD4 + T cells and upregulating the expression of CRISPLD2 [174]. Moreover, Dexamethasone-integrated MSC-based biomimetic liposomes enhance their affinity for polarized macrophages via the LFA-1/ICAM-1 interaction, thereby improving the therapeutic efficacy in rheumatoid arthritis (RA) [175].
In ischemic heart disease, MSCs can reduce the production of inflammatory cytokines, including TNF-α, IL-1β, and IL-6. This finding further demonstrates the potential therapeutic value of MSCs in cardiac repair [176]. In addition, MSCs-EXO carried by specific miRNA may play a regulatory role in the development of inflammation after MI [177, 178]. These anti-inflammatory effects are mainly achieved through the paracrine mechanisms of MSCs and MSCs-EXO [179]. For example, AD-MSCs-EXO carrying miR-93-5p may prevent myocardial damage by inhibiting TLR4-mediated inflammatory responses [180]. Zilun et al. showed that MSCs-EXO carrying miR-181a inhibited c-FOS protein through cell targeting properties and the immunosuppressive effect of miR-81a, thus inhibiting inflammatory response [181]. Neutrophils are inflammatory cells that accumulate in the border area early after MI, so it is essential to investigate the relationship between MSCs and them. Although some studies have shown that BM-MSCs activated by TLR3 and TLR4 in vitro can reduce neutrophil apoptosis through the combined action of IL-6, IL-β and granulocyte macrophage colony-stimulating factor [182]. This does not fully explain why MSCs can inhibit the inflammatory response after myocardial damage. At present, the mainstream view is that MSCs can play a role by inhibiting the activity of neutrophils and accelerating their apoptosis. For example, Dongsheng Jiang et al. suggested that MSCs could phagocytic ICAM1-dependent neutrophils, resulting in a decrease in the number of neutrophils [183]. Other studies have shown that MSCs-EXO can inhibit the excessive spread of inflammation by inhibiting the overactivity of neutrophils [184]. Moreover, the conditioned medium of MSCs have also been found to induce neutrophil apoptosis in vitro [185]. Macrophages are one of the important immune cells that regulate the occurrence and development of inflammation after myocardial damage. Studies have shown that MSCs can accelerate the transformation of M1 macrophages into M2 macrophages in infarct myocardium, it can achieve the purpose of modulating the immunologic environment, accelerating regeneration and inhibiting inflammation [186]. In addition, specifically genetically modified or upregulated MSCs and MSCs-EXO can the duration of inflammation and reduce cardiomyocyte damage by promoting transformation between macrophage subtypes [187, 188]. In summary, it is of great significance to explore how MSCs and MSCs-EXO promote the transformation of M1 macrophages to M2 macrophages and further find the balance between anti-inflammatory and pro- inflammatory.
Promotion of Tissue Regeneration
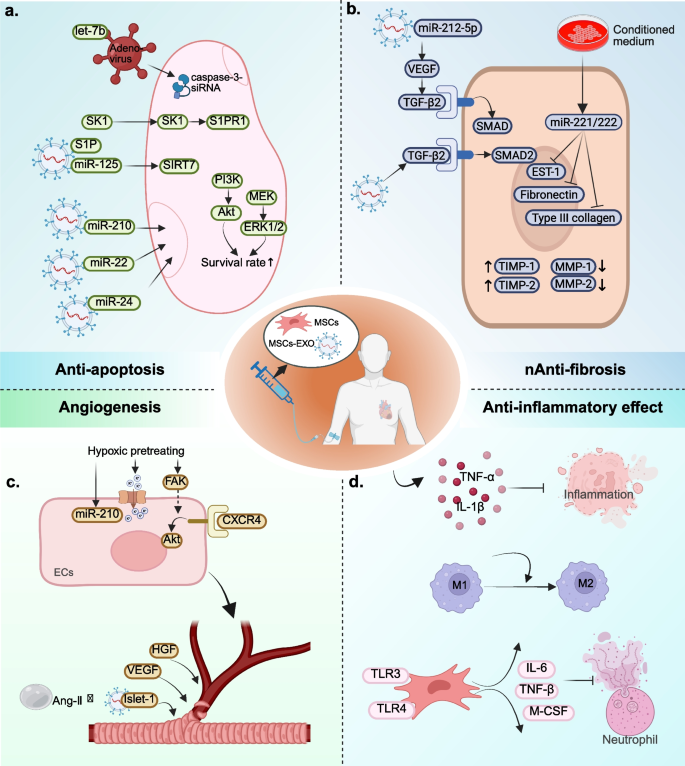
Inhibition of apoptosis, promotion of angiogenesis, targeted differentiation into damaged tissues, and suppression of excessive inflammation constitute four key mechanisms that facilitate tissue regeneration (Fig. 6).
Application of MSCs and MSCs-EXO in MI. a Overview of anti-apoptotic of MSCs and MSCs-EXO; b Overview of anti-fibrosis of MSCs and MSCs-EXO; c Overview of angiogenesis of MSCs and MSCs-EXO; d Overview of anti-inflammatory effect of MSCs and MSCs-EXO. MSCs: mesenchymal stem cells; MSCs-EXO: MSCs-derived exosomes; MI: myocardial infarction
Apoptosis is a complex biological process, and the majority of the mechanisms underlying apoptosis involve a specific type of intracellular enzyme known as the cysteine aspartic acid specific protease (caspase) [189]. The Caspase cascade system plays a crucial role in the induction, transduction and amplification of apoptosis signals within cells. Its activation and function are tightly regulated by a variety of protein molecules and ions including apoptosis inhibitor proteins, Bcl-2 family proteins, calpain, and Ca2+ [190]. At least 7 of the 14 known caspases proteins play a direct role in regulating apoptosis in mammals, while others can also indirectly influence cell death through inflammatory response [191]. Using MI as an example, previous studies have shown that the application of non-selective caspase inhibitors can protect cardiomyocytes from fatal I/R damage in the early stage of MI [192]. Of these known proteins, the activation of caspase-3 is the most crucial in regulating apoptosis following MI. The activation of caspase-3 and the increase of cardiac natriuretic peptides in serum can be detected in non-infarcted areas 1 day after MI and can sustain for up to 4 weeks. At the same time, the Bcl-2/Bax ratio shifted in the direction of pro-apoptotic Bax direction [193]. Chandrashekhar et al. also obtained similar research results through experiments [194]. This suggested that heart failure (HF) may occur in the early stage of MI. Using caspase-3-siRNA to intervene in caspase-3 expression in acute MI (AMI) mice can significantly reduce infarct size and decrease the apoptotic index. Additionally, it improves ventricular function in AMI mice [195]. MSCs transduced with adenovirus-mediated human tissue kallikrein gene can enhance their resistance to hypoxia-induced apoptosis and decrease caspase-3 activity. Moreover, treatment of AMI mice with these cells can improve ventricular function, reduce infarct size, attenuate ventricular remodeling, and promote angiogenesis [196]. Ham et al. found that, compared with conventionally cultured MSCs, mice treated with let-7b-transfected MSCs exhibited improved left ventricular function and increased microvascular density. This effect is achieved by protecting the transplanted MSCs from apoptosis and autophagy through direct targeting of the caspase-3 signaling pathway by let-7b [197]. These studies indicate that MSCs can reduce cardiomyocyte apoptosis after MI by decreasing the activity of caspase-3.
Not only MSCs have anti-apoptotic effect, but MSCs-EXO can also reduce the oxidative stress after MI and improve the anti-apoptotic ability of cardiomyocytes in the peri-infarction area. Regardless of the choice of intramuscular injection [198, 199], intra-coronary injection [200], intravenous injection (IT) [201, 202] or intracardiac injection [203], MSCs-EXO can play a role in reducing the apoptosis of endogenous cardiomyocytes. Pretreated or genetically modified MSCs-EXO have better anti-apoptotic ability than untreaded or unmodified MSCs-EXO. For example, MSCs under anoxia can release exosomes enriched with miR-210 and reduce cardiomyocyte apoptosis after MI [204]. MSCs-EXO can ameliorate myocardial injury and reduce apoptosis after MI by activating S1P/SK1/S1PR1 signaling and promoting M2 macrophage transformation [205]. Zhang et al. [206] also showed that the miR-24 released level of BM-MSCs-EXO hypoxic preconditioning was significantly higher than that of normal-oxygen pretreating of BM-MSCs. After the injection of hypoxic BM-MSCs-EXO, the level of miR-24 in AMI mice showed significant differences. The MI size was reduced and the left ventricular function improved. Other studies have shown that genetically modified MSCs-EXO with miR-125b can target SIRT7 binding to prevent myocardial injury and apoptosis reduced by I/R [207]. Luo et al. showed that AD-MSCs-EXO with overexpression of miR-126 could effectively reduce cardiomyocyte apoptosis in myocardial border area and prevent myocardial damage [208]. The above studies indicate that MSCs-EXO exhibits a diverse array of delivery pathways, a remarkable homing capacity, and significant anti-apoptotic properties. Furthermore, they provide a valuable research foundation for the potential clinical application use of MSCs-EXO in the treatment of cardiomyocyte apoptosis after MI.
During MI-induced cell apoptosis, not only does oxidative stress play a role, but there is also a loss of ATP and NADH. Treatment with MSCs-EXO increased ATP and NADH levels, reduced oxidative stress, and enhanced activation of the PI3 K/AKT pro-survival signaling pathway. Additionally, MSCs-EXO treatment decreased the phosphorylation of c-JNK, a key activator of pro-apoptotic signaling [209]. This suggests that MSCs-EXO reduces apoptosis at least in part by restoring the bioenergetics of target cardiomyocytes and reducing oxidative stress.
The inflammatory response has been comprehensively detailed in the preceding Sect.” Immunomodulatory and anti-inflammatory“and will not be reiterated here.
The new small capillaries are hollow tubular structures surrounded by ECs which are supported by pericytes. The outsourced basal cells and extracellular matrix (ECM) can further enhance the structural stability of small capillaries [210]. In the physiological state, ECs maintain inactive and the surrounding basal cells and pericytes also maintain the normal order of ECs to perform its functions. However, when the environmental and mechanical stresses in the injured tissue change, these stable structures are destroyed [211]. Preexisting ECs in the infarct margin area will produce initial new capillaries by budding [146]. The budding process begins with the breakdown of basement membrane by proteolytic enzymes and the isolation of pericytes. After that, ECs can be activated. With the help of VEGF and other factors, activated ECs escaped from the original capillaries and invaded the temporary scaffold matrix to participate in budding. Their cell characteristics and molecular expression profiles are altered, and they will differentiate into tip and stalk cells with different morphology and functions [212, 213]. The tip cells have a large number of filamentous feet, which are mainly responsible for migration and coordinating the direction of branch formation. During bud elongation, stalk cells proliferate and form new primitive capillary lumen with the proliferation of stem tip cells [213, 214]. The stable budding then begins to transform into mature blood vessels and fuse with existing capillaries. The ECM also gathers around the nascent original capillaries and recruits pericytes to cover the ECs [215]. Ultimately, the blood vessels achieve stability and blood flow normalizes.
Homing ability is the basis for MSCs to participate in angiogenesis [216]. MSCs can migrate and adhere to ischemic tissues through differentiation, direct contact or paracrine, and play a role in promoting angiogenesis [217, 218]. For example, hypoxia and reoxygenation can accelerate the state recovery of MSCs, improve the expression of pro-survival genes and various nutritional factors in MSCs, and indirectly increase the homing ability and angiogenesis of MSCs [219, 220]. In addition, hypoxia pretreating of BM-MSCs can enhance their migration capacity by activating Kv2.1 channels and FAK [221]. The above studies indicate that hypoxia pretreating can improve the survival rate of transplanted MSCs in the infarct junction and promote homing ability and angiogenesis. It provides a good experimental basis and theoretical basis for us to further explore the relationship and mechanism between hypoxia pretreating, homing ability and angiogenesis of MSCs.
In addition to hypoxia, several genes related to angiogenesis can also be directly transferred into MSCs to enhance the angiogenic ability of MSCs. Using MI as an example, MSCs transfected with HGF or VEGF can promote angiogenesis and reduce fibrosis, thereby improving ventricular function in MI-induced porcine models [222]. Compared with MI mice injected with ordinary MSCs, MI mice injected with Ang-1 overexpressing MSCs had a better effect of angiogenic (approximately 11–35% more than the control group) [223]. Other studies have shown that basic fibroblast growth factor can control the migration of MSCs and improve the integration of MSCs with ECs to promote angiogenesis [224, 225]. These studies collectively suggest that appropriate genetic engineering of MSCs is essential to enhance their angiogenic potential.
The regenerative capacity of MSCs is also associated with their differentiation potential. In addition to osteogenic, chondrogenic, and adipogenic differentiation potential, MSCs are also capable of differentiating into steroidogenic cell lineages [226], myogenic cells [227], hepatocyte-like cells [228], and cardiomyocytes [229]. However, whether MSCs can differentiate into ECs is still controversial in current studies. At present, only a few studies support that MSCs can differentiate into ECs-like cells [230,231,232]. Most scholars believe that MSCs and MSCs-EXO can promote angiogenesis mainly because they can interact directly with damaged ECs or in paracrine form [233, 234]. After MSCs come into direct contact with the damaged ECs, tunneling nanotube-like structures are formed. This structure allows MSCs to move frequently and almost unidirectionally towards the damaged ECs, thereby resulting in the rescue of aerobic respiration and protection of ECs from apoptosis [235]. In the paracrine studies of MSCs and MSCs-EXO for the promotion of tissue regeneration, MSCs-EXO has been the most extensively investigated in recent years. The current research mainly focuses on the following three aspects: (1) Combining MSCs-EXO with some new molecular materials can improve the therapeutic effect of MSCs-EXO. For example, if MSCs-EXO overexpressing Islet-1 is combined with Ang-1 gel, the retention of MSCs-EXO in the heart can be increased, and its anti-apoptotic effect can be further enhanced to promote angiogenesis, to enhance the anti-apoptotic effect and promoting angiogenesis [236]. After the injection of magnetic iron oxide nanoparticles with MSCs-EXO mixed reagents into MI’s heart, the inflammatory period can be transformed into a repair period in advance under magnetic guidance. This results in a reduction of apoptosis, an alleviation of fibrosis, the promotion of angiogenesis, and the recovery of ventricular function [237]. After all, MSCs-EXO mixed new materials are exogenous implants, and whether they have adverse effects on the human body still needs long-term follow-up observation, so we still need to be cautious about their use. (2) MSCs-EXO (or pretreating of MSCs-EXO) were modified by cell engineering will promote angiogenesis and tissue repair. For example, up-regulation of CXCR4 expression in MSCs-EXO can promote angiogenesis through AKT signaling pathway [238]. MSCs pretreated with atorvastatin can improve the survival rate of ECs and increase the expression of VEGF and ICAM-1 gene to improve the therapeutic effect [239]. However, it is unclear whether modification or pretreatment affects the genetic properties of MSCs-EXO, so its clinical application remains to be further investigated. Optimizing the delivery mode of MSCs-EXO can improve the utilization rate and promote angiogenesis. The emergence of new delivery methods, such as intra pericardial delivery [240] and exosome spray [241], has changed the traditional concept that MSCs-EXO can only be used by injection. These advancements may pave the way for new therapeutic strategies and could become a major focus of future research and development in the field of MSCs-EXO.
Therapeutic applications in disease models
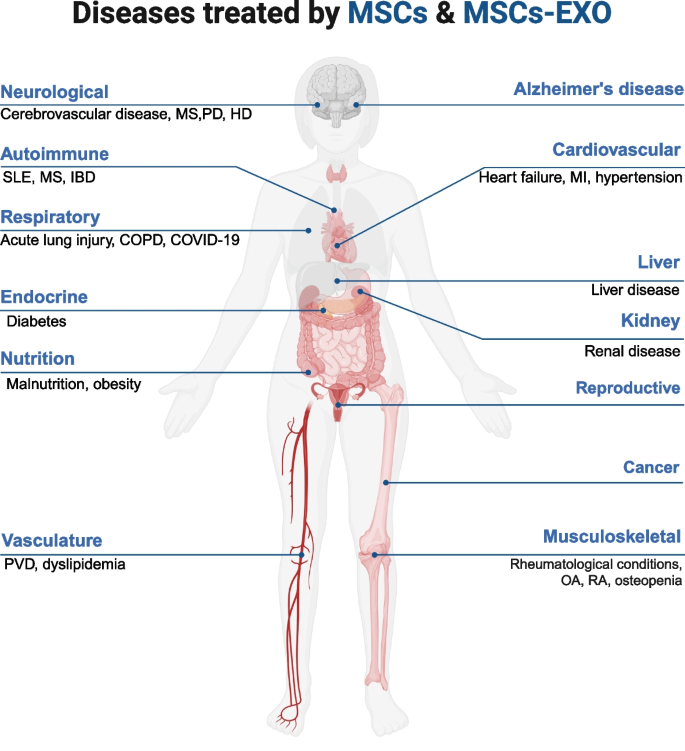
Currently, MSCs and MSCs-EXO have been extensively utilized in clinical studies owing to their promising therapeutic effects. This section will summarize their applications in CVDs, neurological disorders, ADs, MSDs, and other conditions (Fig. 7).
Overview of MSCs and MSCs-EXO applications in Disease Models. MSCs and MSCs-EXO can exert therapeutic effects in a wide range of diseases, including those affecting the nervous system, respiratory system, circulatory system, digestive system, urinary system, reproductive system, musculoskeletal system, immune system, and many others. MSCs: mesenchymal stem cells; MSCs-EXO: MSCs-derived exosomes; MS: multiple sclerosis; PD: parkinson’s disease; HD: huntington’s disease; SLE: systemic lupus erythematosus; IBD: inflammatory bowel disease; COPD: chronic obstructive pulmonary disease; PVD: peripheral vasculature disease; MI: myocardial infarction; OA: osteoarthritis; RA: rheumatoid arthritis
Therapeutic applications in CVDs
CVD, a broad term encompassing disorders that affect the heart and blood vessels, is a significant cause of morbidity and mortality, posing a major threat to human health and well-being. A retrospective study from 204 countries reported that the number of patients with CVD increased from 271 to 523 million, and the number of deaths with CVD increased from 12.1 million to 18.6 million between 1990 and 2019 [242]. This is a huge challenge for the global health system. MI is an important component of CVD. MI refers to the condition where reduced coronary blood flow, due to various causes, results in ischemia and hypoxia of cardiomyocytes, leading to necrosis in the myocardial region supplied by the affected coronary artery [243]. MI can be classified into AMI and chronic MI (CMI) based on the course and nature of the disease. The primary cause of AMI is acute thrombosis. When a coronary artery is abruptly occluded by an acutely dislodged thrombus, myocardial necrosis can occur within minutes to hours. However, CMI is a long-term pathophysiological process characterized by the gradual expansion of coronary atherosclerotic plaques, leading to progressive luminal narrowing and resultant myocardial ischemic injury. The exact timeframe for CMI development remains variable [244]. Cardiomyocyte apoptosis and subsequent inflammatory response will lead to local tissue fibrosis, scar tissue formation and poor ventricular remodeling. They can have adverse effects on the regeneration of the heart, and some patients may progress to HF or even death [245]. All the above pathophysiological processes occur throughout the progression of the disease, both in the AMI and CMI. Therefore, it is of great significance to explore a new biologic therapy to alter or reverse these pathophysiological processes.
In recent years, MSCs and MSCs-EXO have emerged as promising therapeutic modalities in the clinical treatment of CVDs, including MI. We have summarized the clinical application of MSCs in Table 1 and found that BM-MSCs are the most commonly utilized type for the treatment of MI, followed by AD-MSCs and UC-MSCs. In contrast, DP-MSCs are primarily used in basic research, with clinical studies predominantly focusing on pulp and bone regeneration following tooth injury. Although a large number of preclinical studies have shown that MSCs have a positive effect on the treatment of MI, the results of existing clinical trials have been polarized. For example, after autologous BM-MSCs transplantation in 10 AMI patients who received standard therapy, Bodo et al. found that, compared with MI patients receiving standard therapy, those in the cell therapy group exhibited a significant reduction in the percentage of infarct size relative to the total left ventricular area and a significant increase in the velocity of ventricular wall motion in the border zone [246]. This suggests that BM-MSCs may possess reparative effects on cardiomyocytes following MI. According to another study involving 69 patients with AMI who were randomized to receive either BM-MSCs or saline following percutaneous coronary intervention (PCI) within 12 h of symptom onset, left ventricular function was significantly improved in the BM-MSCs treatment group (n = 34) [247]. With the deepening of research, more and more clinical studies have confirmed the safety of MSCs in clinical application and its protective effect on myocardial tissues after MI [248, 249]. However, some studies have shown that MSCs have a limited therapeutic effect on MI. For example, Wollert et al. [250] showed that patients treated with MSCs did not show a significant increase in left ventricular ejection fraction (LVEF) compared to controls. In addition, according to another study in Norway, Patients in the acute stage of MI did not significantly improve left ventricular function after intra coronary injection of BM-MSCs [251]. Other studies have demonstrated that, while patients treated with MSC injections exhibited improved LVEF, reduced left ventricular end-systolic volume, enhanced stroke output, and smaller MI scar areas, there were no significant differences in overall mortality or cardiovascular mortality compared to those receiving conventional therapy [252]. Although the above data indicate that MSCs are not ideal for the clinical treatment of MI, most studies show that the effect of MSCs on the clinical treatment of MI cannot be ignored, compared with untreated group, LVEF and ventricular function were improved significantly. We analyzed the reasons for the large heterogeneity in the above studies as follows: (1) The total number of participants in the study was too small for statistical analysis; (2) Different amounts of imported MSCs or different injection sites lead to heterogeneity of treatment outcomes [253]; (3) Different preparation techniques and algebras of MSCs lead to different therapeutic effects; (4) There are differences in the treatment time window.
It is noteworthy that our review of clinical studies revealed that the majority of trials utilized coronary artery administration, whereas IT and transendocardial stem cell injection (TESI) were employed in only a minority of cases. In recent years, stem cell pericardial implantation technology has emerged as a novel approach and has gradually garnered attention. Although coronary artery administration remains the predominant method for stem cell therapy in MI, significant variability in clinical outcomes persists. A study by HOPP et al. demonstrated that, compared with the control group receiving sham injections, the experimental group of 15 patients who received intra-coronary injections of BM-MSCs did not exhibit significant improvements in cardiac function, and the reduction in infarct size was not statistically significant [274]. Although a few studies have indicated that MSCs treatment may be ineffective, it is important to note that no serious adverse reactions have been observed following autologous transplantation of MSCs. This suggests that, despite the controversy surrounding its efficacy, the safety of MSCs in clinical applications is well-established. In addition to autologous transplantation, MSCs are also suitable for allotransplantation. While allogeneic MSCs exhibit low immunogenicity, immune rejection may still occur in some cases. For instance, it has been demonstrated that recipients of completely mismatched allogeneic MSCs may develop memory T cells that can trigger immune rejection [285]. The survival time of allogeneic MSCs post-transplantation is typically shorter than that of autologous MSCs, which may influence therapeutic outcomes. Additionally, MSCs derived from different tissue sources exhibit variations in biological properties, culture characteristics, and differentiation potential, potentially leading to differences in therapeutic efficacy.
Although MSCs-EXO has shown certain potential in clinical studies of atomized inhalation in the treatment of novel coronavirus pneumonia and other diseases [286], its application in clinical studies is still insufficient compared with MSCs. The administration route and dosage optimization of MSCs-EXO need to be further explored. In addition, MSCs-EXO is rapidly cleared in the body, has poor stability, and is susceptible to temperature, pH and mechanical stimulation [287]. Therefore, it is crucial to develop effective delivery systems to enhance their persistence in vivo and to establish appropriate preservation methods to ensure their efficacy and stability. Future research should further investigate the clinical applications of MSCs and MSCs-EXO to fully realize their therapeutic potential.
Therapeutic applications in neurological disorders
Neurological disorders rank fourth in the global disease burden [288]. Its common clinical manifestations include sensory and motor dysfunction, as well as impaired cognitive function. Given the limited regenerative capacity of nerve cells following damage, these conditions often result in long-term or even lifelong challenges for patients [289]. In therapeutic interventions, the timely and effective repair of injured neurons is crucial for maintaining normal neural function. MSCs represent an ideal cell source for neural regeneration and repair. By secreting a diverse array of bioactive factors, including miRNAs, proteins, and growth factors, MSCs not only promote neuronal survival and nerve regeneration but also inhibit inflammatory responses, thereby significantly enhancing neural function [290].
In recent years, MSCs and MSCs-EXO have been widely used as an alternative therapy for the clinical treatment of neurological diseases. We summarized the recent clinical applications of MSCs and MSCs-EXO in neurological diseases in Table 2. Spinal muscular atrophy (SMA) is a neurodegenerative disorder resulting from mutations in the SMN1 gene [291]. In patients with SMA, the capacity of MSCs to cross the blood–brain barrier can be leveraged to maximize the therapeutic efficacy of IT [292]. Meanwhile, intravenous injection (IV) is more appropriate for targeting organs such as the lungs, spleen, liver, bone marrow, and thymus, which are characterized by their propensity for cellular diffusion [293]. Studies have demonstrated that IV of MSCs promotes the release of neurotrophic factors and cytokines, facilitating their entry into pathological tissues. This approach has been shown to significantly improve the tibial nerve motor amplitude response in patients with SMA, thereby demonstrating the tolerability and safety of MSCs in SMA treatment [294].
MSCs treated by IV injection in combination with IT is considered a promising therapeutic strategy for treating neurological disorders. Lu Z et al. [327] investigated the use of long-established IV administration in combination with IT infusion of UC-MSCs for treating relapsing–remitting multiple sclerosis (RRMS) and neuromyelitis optica (NMO). During a 10-year follow-up of patients with NMO spectrum disorder (NMOSD), no adverse reactions indicative of intolerance, such as tumor formation or peripheral organ/tissue disease, were observed in either RRMS or NMOSD patients. This suggests that the combination of IV and IT administration is safe and feasible. Additionally, the study by Jamali F et al. [317] found that in patients with multiple sclerosis (MS) treated with UC-MSCs, those receiving two doses of IV and IT administration exhibited significantly greater improvement in clinical symptoms compared to the control group, which received a single dose of IV and IT treatment. The downregulation of inflammation-related genes, including TNF-α, TAP-1, and miR-142, indicates that the combination of IV and IT administration is effective. However, while the current study supports the effectiveness of combining IV and IT administration of MSCs for neurological disorders, further clinical trials are necessary to verify its long-term effects and determine the optimal dosing regimen.
Therapeutic applications in ADs
ADs constitute a complex class of disorders whose etiology remains incompletely understood. Although there may be some similarities in the clinical presentation of different ADs, each condition exhibits its own distinct characteristics [328]. ADs are typically characterized by the activation of autoreactive T and B cell clones [329]. In these conditions, the immune system erroneously targets the body’s own normal cells, resulting in a diverse array of symptoms and pathological changes.
SLE is one of the most prevalent ADs. Recent studies have demonstrated that MSCs and MSCs-EXO exhibit potent immunomodulatory effects in the treatment of SLE, influencing immune-related cells such as T cells, B cells, NK cells, and macrophages. The immunomodulatory properties and multidirectional differentiation potential of MSCs and MSCs-EXO make them valuable tools for treating ADs. However, due to concerns regarding the carcinogenic risks associated with genetic mutations, genetic instability, and excessive proliferation and differentiation of MSCs, MSCs-EXO is considered a more favorable option for future SLE treatments [330]. Studies have demonstrated that in a non-obese diabetic (NOD) rat model, treatment with AD-MSCs-EXO and BM-MSCs-EXO can effectively repair damaged neurons and astrocytes, specifically inhibit the overactivation of T and B lymphocytes, and mitigate autoimmune damage to islet cells. This results in improved cognitive function and reduced blood glucose levels [331]. We summarized the clinical application of MSCs and MSCs-EXO in ADs in the past 5 years (Table 3). In the trial conducted by Kamen DL et al. [350], six women with refractory SLE were treated with MSCs at a dose of 1 × 106 cells/kg. The results indicated a downward trend in autoantibody levels, while the GARP-TGF-β index in B-cell serum increased. These findings suggest that MSCs exert a systemic immunomodulatory effect consistent with the observed clinical treatment outcomes. In a triple-blind, placebo-controlled, randomized trial conducted by Fernandez et al. [351], patients with secondary progressive multiple sclerosis (SPMS) were followed for 12 months after infusion of autologous AD-MSCs. Only one serious adverse event (a urinary tract infection, deemed unrelated to the study treatment) was observed. These findings suggest that the use of AD-MSCs in treating SPMS is both safe and feasible. However, data from this trial did not yield significant results for potential markers of therapeutic effect in immune testing. Therefore, larger studies are necessary to further investigate the therapeutic benefits of MSCs. In the trial conducted by Chun S et al. [352], seven patients with lupus nephritis (LN) received an intravenous infusion of 3.0 × 106 cells/kg BM-MSCs, with no dose-limiting toxicity observed. Additionally, in animal models, BM-MSCs distribution in the kidneys persisted until day 7. These findings demonstrated the safety and tolerability of BM-MSCs in LN patients and identified 3.0 × 106 cells/kg as the maximum tolerated dose for this patient population. Given the relatively limited number of patients with ADs currently treated with MSCs and MSCs-EXO, the existing results may be subject to bias. Therefore, it is essential to conduct further experimental validation in larger patient populations.
Therapeutic applications in MSDs
MSDs is a common group of diseases affecting human health, usually involving muscles, bones, tendons, ligaments, cartilage and related tissues. The main clinical symptoms include pain, stiffness, swelling and limited motor function [353]. This condition is often attributed to a variety of factors, including repetitive strain, poor posture, chronic stress, or traumatic injury [354]. OA and RA are among the most prevalent MSDs. OA can lead to intractable pain, limit daily activities, and significantly reduce patients’quality of life, although it is not inherently a fatal condition. RA, on the other hand, is a systemic autoimmune disease that can affect multiple joints throughout the body and is not secondary to OA. Currently, clinical treatment primarily focuses on pain relief, rehabilitation, or surgical intervention; however, these approaches are not effective in preventing the progression from OA to RA [355]. As a cell-based therapy, MSCs and MSCs-EXO demonstrate significant potential in the treatment of MSDs. MSCs have the capacity to differentiate into osteoblasts and chondrocytes, and they promote cartilage repair and regeneration through the secretion of various bioactive factors, including growth factors and cytokines [356]. MSCs-EXO can promote chondrocyte proliferation, inhibit cell apoptosis, and enhance the synthesis of cartilage matrix [357]. These characteristics underscore the significant potential of MSCs and MSCs-EXO in the cellular therapy of bone and cartilage diseases.
We have summarized the clinical applications of MSCs and MSCs-EXO in MSDs over the past five years (Table 4). Our analysis reveals that the most common method for administering MSCs or MSCs-EXO in the treatment of OA patients is direct intra-articular injection into the affected joint. This method allows the therapeutic agents to act directly on the damaged area, thereby enhancing treatment efficacy and minimizing side effects. While several alternative modes of administration are also under investigation, intra-articular injection currently remains the preferred strategy. Kim KI et al. [342] reported that 125 patients with OA received intra-articular injections of 1 × 108 AD-MSCs. Compared to the control group, the injection group demonstrated significant meniscus regeneration and a reduction in knee pain. Chen CF et al. [341] also reported that six patients with knee OA received intra-articular injections of 6.7 × 106 autologous AD-MSCs. The results demonstrated that patients experienced a significant reduction in knee pain, no serious adverse reactions were observed, and the quality of articular cartilage improved. Additionally, studies have found that when platelet-rich plasma (PRP) is administered in conjunction with MSCs, PRP significantly enhances the intra-articular retention time and survival rate of MSCs in an anti-inflammatory environment [358]. In the study by Lamo-Espinosa JM et al. [346], patients received intra-articular injections of 100 × 106 autologous BM-MSCs in combination with PRP. Compared to the control group, which received only PRP injections, the experimental group demonstrated significant improvements in joint mobility function without any adverse reactions. A study by Vij R et al. [348] demonstrated that a single intravenous administration of autologous AD-MSCs significantly improved joint function in RA patients and, to some extent, reduced the levels of the inflammatory marker C-reactive protein (CRP). The treatment had minimal impact on renal and liver function, indicating that AD-MSCs are both safe and effective for treating RA. In patients with chronic low back pain, a single intra-discal injection of AD-MSCs combined with hyaluronic acid significantly alleviated pain symptoms and the imaging studies demonstrated a marked reduction in disc herniations [332]. Although these studies provide positive evidence, further large prospective, placebo-controlled trials are needed to validate the long-term efficacy and optimal dosing regimen of MSCs and MSCs-EXO.
Therapeutic applications in some other diseases
In addition to the treatment of these diseases, MSCs have also been widely used in diseases such as COVID-19, acute lung injury, chronic obstructive pulmonary disease (COPD), cirrhosis and CKD (Table 5) [370]. For acute lung injury, MSCs can promote the repair of alveolar epithelial cells by secreting a variety of growth factors and cytokines [371]. In COPD, MSCs reduce airway inflammation and reduce airway remodeling [372]. In the treatment of cirrhosis, MSCs can promote liver cell regeneration and reduce liver inflammation and fibrosis [373]. In CKD, MSCs can inhibit renal fibrosis and improve renal function. CKD is characterized by structural or functional abnormalities of the kidneys, typically persisting for more than three months. It can be caused by a variety of conditions, with diabetic nephropathy being the most common etiology. Diabetic nephropathy not only represents the leading cause of CKD but is also one of the most frequent complications associated with diabetes [374]. Hyperglycemia can trigger a cascade of pathophysiological events leading to glomerular and tubulointerstitial fibrosis, characterized by podocyte damage or loss and mesangial cell hypertrophy [375]. Therefore, glycemic control is paramount in the management of diabetic nephropathy. MSCs can release anti-inflammatory factors such as HGF and prostaglandin E2 via paracrine mechanisms to mitigate inflammatory responses, reduce the expression of pro-fibrotic molecules, and decrease collagen and fibronectin deposition. This ultimately ameliorates renal fibrosis and promotes the regeneration and functional recovery of islet beta cells, thereby mitigating kidney damage caused by hyperglycemia [376]. MSCs-EXO can ameliorate the symptoms of diabetic nephropathy by regulating autophagy, inhibiting fibrosis, and alleviating inflammation. For instance, BM-MSCs-EXO can induce autophagy via the mTOR signaling pathway, significantly increasing the expression of LC3 and Beclin-1 in renal tissue while reducing mTOR expression levels. This ultimately improves renal function and repairs pathological damage [377]. Furthermore, MSCs-EXO can exert an anti-apoptotic effect by inhibiting the expression of TGF-β1, maintaining the expression of tight junction proteins, and preventing the epithelial-mesenchymal transition (EMT) of renal tubular epithelial cells [378]. As research continues to advance, the application of MSCs in the treatment of an expanding range of diseases will be further optimized, offering new hope to patients.
Conclusion and prospect
MSCs and MSCs-EXO are extensively utilized in the treatment of various common clinical conditions, particularly in CVDs, neurological disorders, ADs, and MSDs, with some studies achieving remarkable research outcomes. Although MSCs may pose carcinogenic risks such as gene mutation, genetic instability, and excessive proliferation and differentiation during treatment, and their biological activity can diminish in hypoxic microenvironments, thereby affecting therapeutic efficacy, MSCs still demonstrate significant potential as a promising treatment for regenerative medicine and tissue engineering applications.
MSCs-EXO are paracrine products of MSCs and can compensate for certain limitations of MSCs through effective intercellular communication. As a novel form of non-cellular therapy, MSCs-EXO exhibit unique advantages and characteristics in disease treatment. Although MSCs-EXO exhibit greater versatility compared to MSCs, their current clinical application is significantly more limited. The reasons for this include the following: first, the preparation and purification processes for MSCs-EXO have not yet met the standards required for clinical use; second, the storage and transportation of MSCs-EXO necessitate more stringent conditions; third, the optimal dosages of MSCs-EXO for various diseases are still under investigation. These factors collectively constrain the clinical application of MSCs-EXO.
The extensive body of existing clinical data unequivocally demonstrates the efficacy of MSCs and the utilization of MSCs-EXO in the treatment of various clinical diseases. These treatments exhibit high feasibility and safety profiles. Although studies have predominantly demonstrated positive outcomes, heterogeneity is observed in certain studies [255, 258, 261, 266, 271, 274, 295, 297, 304,305,306, 308, 310, 314, 324, 335, 338, 351]. After an in-depth summary of the above negative results, we found that the main reasons for these heterogeneous results included the limited number of patients included in the study and the insufficient sample size [258, 261, 297, 304,305,306, 308, 348, 351]. This has led to significant individual differences, such as variations in age, severity of the disease, measurement results [308], thus making it impossible to conduct in-depth multivariate analysis [297]. Furthermore, the lack of corresponding control groups for comparison [304], and a large number of unexpected placebo effects [295, 308], may had an impact on the differences in the results. In addition, due to the limited follow-up time, negative results may occur in the short term [274], and the accurate assessment of some evaluation indicators requires a longer follow-up time. In preclinical research, intervention measures can often be carried out at the early stage of disease development. However, due to the limitations of complex factors such as subject screening and experimental preparation, clinical research usually can only implement intervention measures in the late stage of the disease [324]. Intervention in the early stage of the development of certain diseases, such as the application of MSCs for treatment in the early stage after MI, may cause tissue edema in the myocardial stroma due to the recanalization of the infarct-related arteries, thereby affecting the therapeutic effect [255, 258]. In addition, the in vitro culture time of MSCs is usually long, and the long-term in vitro culture increases the risk of cell contamination [255]. It is worth noting that MSCs cells are relatively large in size, have poor motility and migration capabilities, and are prone to clumps, which may lead to vascular obstruction [255]. Meanwhile, factors such as the selection of dosage during the treatment of MSCs [266], potential cell-mediated effects [271], and biological variability [338] may lead to unsatisfactory therapeutic effects.
To summarize, the heterogeneity observed in MSCs and MSCs-EXO during clinical applications can primarily be attributed to the following factors: (1) Donor variability: The origin of MSCs and MSCs-EXO may vary due to differences in donor age, health status, gender, and genetics. (2) Isolation methods: Different isolation methods are required for various sources of MSCs. During the isolation process, contamination from other cell types, residues, or chemical enzymes may affect the growth and proliferation of target MSCs. (3) Culture conditions: Variations in culture environments, culture conditions, consumable materials, and cell passages of MSCs also contribute to heterogeneity in clinical trials. (4) Purification techniques: The use of different collection methods to obtain clinical-grade, highly purified MSCs and MSCs-EXO is a critical factor influencing the effectiveness of clinical therapy. (5) Transportation conditions: Proper transportation methods are essential to maintain the activity of MSCs and MSCs-EXO during transit. (6) Immunogenicity: Although MSCs exhibit low immunogenicity under normal conditions, disease-induced changes in the microenvironment and inflammation can lead to the upregulation of MHC II molecules on their surface, potentially causing immune rejection and thereby affecting therapeutic outcomes. (7) Injection parameters: Differences in site, frequency, type, quantity, and source of the injected MSCs and MSCs-EXO significantly impact therapeutic efficacy. (8) Disease characteristics: The type and severity of the disease also significantly influence the therapeutic efficacy of stem cells and exosomes. (9) Administration timing: The variations in the administration timing of MSCs and MSCs-EXO, as well as disparities in follow-up durations, may also contribute to differences in therapeutic efficacy. (10) Included patients’ numbers: The limited number of patients enrolled in the study, coupled with an insufficient sample size, contributed to the observed heterogeneity. (11) Placebo: The rationality of placebo selection. (12) Others: Cell-mediated effects, biological variability, injection-site edema, cell contamination, and volume changes, among other factors.
Although there are currently no standardized processes for the isolation, characterization, and administration of MSCs and MSCs-EXO, the disparities in clinical outcomes caused by these factors can be mitigated through existing technologies. Firstly, when selecting the type of MSCs, priority can be given to MSCs with stable biological characteristics (e.g., UC-MSCs), and gene-editing techniques can be employed to minimize donor-to-donor variability. However, this may give rise to certain medical ethics concerns. Secondly, stringent donor-screening criteria should be established, with a preference for young and healthy donors. An efficient and well-defined preparation process should be applied, utilizing advanced extraction techniques such as ultracentrifugation and robust quality control measures like Western blot analysis to ensure the production quality of MSCs and MSCs-EXO. Standardization of cell characterization can also be achieved by identifying specific surface markers. In the early stages of clinical application, its safety and efficacy can be validated through multi-center, large-scale clinical trials, thereby facilitating the translation of this technology from the laboratory to the clinical setting.
The development of engineered exosomes may address the challenges posed by the aforementioned factors to a certain extent. The conjugation of homing peptides, which possess cell or tissue-targeting capabilities, with MSCs-EXO significantly enhances the delivery efficiency of MSCs-EXO. This enables more precise action on specific lesion sites, thereby improving therapeutic efficacy. In addition, by conjugating homing peptides, MSCs-EXO can overcome the limitations of low survival rates and insufficient targeting capabilities observed in traditional cell therapies. Simultaneously, they avoid the risks associated with live-cell treatments, thus further enhancing their applicability in disease management [387]. However, several issues still require further resolution, including the standardized production and quality control of exosomes, as well as the binding stability between homing peptides and exosomes. MSCs-EXO can facilitate complex intercellular signal transduction processes via the transfer of bioactive factors such as proteins and nucleic acids, mitochondrial intercellular transfer, protein–protein interactions, receptor-ligand interactions, and other targeted mechanisms. They not only exhibit anti-inflammatory and immunomodulatory properties but also promote the generation and repair of tissues such as blood vessels and nerves while inhibiting cell apoptosis. During the preclinical experimental phase, MSCs-EXO demonstrated significant synergistic protective effects on the heart, nervous system, immune system, and overall body metabolism.
Therefore, further basic research is essential to elucidate the underlying treatment mechanisms and to enhance both efficacy and stability. Additionally, larger clinical trials are necessary to establish standardized protocols for the isolation, culture, preservation, and application of MSCs and MSCs-EXO. These efforts will contribute to achieving greater effectiveness and stability in the therapeutic application of MSCs and MSCs-EXO.
Data availability
Not applicable.
References
-
Ramalho-Santos M, Willenbring H. On the origin of the term “stem cell.” Cell Stem Cell. 2007;1(1):35–8. https://doi.org/10.1016/j.stem.2007.05.013.
-
Friedenstein AJ, Piatetzky S II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–90.
-
Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50. https://doi.org/10.1002/jor.1100090504.
-
Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323–48. https://doi.org/10.1007/s00018-019-03125-1.
-
Bonucci E. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967;20(1):33–50. https://doi.org/10.1016/s0022-5320(67)80034-0.
-
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. https://doi.org/10.1038/nrm.2017.125.
-
Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. https://doi.org/10.1146/annurev-biochem-013118-111902.
-
Stegmayr B, Brody I, Ronquist G. A biochemical and ultrastructural study on the endogenous protein kinase activity of secretory granule membranes of prostatic origin in human seminal plasma. J Ultrastruct Res. 1982;78(2):206–14. https://doi.org/10.1016/s0022-5320(82)80024-5.
-
Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74(5):1844–51.
-
F Z, J G, Z Z, Y Q, G W, M D, et al. Mesenchymal stem cell-derived exosome: A tumor regulator and carrier for targeted tumor therapy. Cancer letters. 2022;526:29–40. https://doi.org/10.1016/j.canlet.2021.11.015.
-
Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. J Hepatol. 2016;65(1):213–21. https://doi.org/10.1016/j.jhep.2016.03.004.
-
Ibrahim A, Marbán E. Exosomes: Fundamental Biology and Roles in Cardiovascular Physiology. Annu Rev Physiol. 2016;78:67–83. https://doi.org/10.1146/annurev-physiol-021115-104929.
-
Williams T, Salmanian G, Burns M, Maldonado V, Smith E, Porter RM, et al. Versatility of mesenchymal stem cell-derived extracellular vesicles in tissue repair and regenerative applications. Biochimie. 2023;207:33–48. https://doi.org/10.1016/j.biochi.2022.11.011.
-
Missoum A. Recent Updates on Mesenchymal Stem Cell Based Therapy for Acute Renal Failure. Curr Urol. 2020;13(4):189–99. https://doi.org/10.1159/000499272.
-
Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4(5):267–74.
-
Miao C, Lei M, Hu W, Han S, Wang Q. A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res Ther. 2017;8(1):242. https://doi.org/10.1186/s13287-017-0697-9.
-
Cakouros D, Gronthos S. Epigenetic Regulation of Bone Marrow Stem Cell Aging: Revealing Epigenetic Signatures associated with Hematopoietic and Mesenchymal Stem Cell Aging. Aging Dis. 2019;10(1):174–89. https://doi.org/10.14336/ad.2017.1213.
-
Zou XF, Zhang BZ, Qian WW, Cheng FM. Bone marrow mesenchymal stem cells in treatment of peripheral nerve injury. World J Stem Cells. 2024;16(8):799–810. https://doi.org/10.4252/wjsc.v16.i8.799.
-
Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129(3):163–73. https://doi.org/10.1016/j.mad.2007.12.002.
-
Lee JS, Kim SK, Cha JK, Jung BJ, Choi SB, Choi EY, et al. Novel Technique for Isolating Human Bone Marrow Stem Cells Using Hyaluronic Acid Hydrogel. Tissue Eng Part C Methods. 2016;22(10):941–51. https://doi.org/10.1089/ten.TEC.2016.0214.
-
Torre ML, Lucarelli E, Guidi S, Ferrari M, Alessandri G, De Girolamo L, et al. Ex vivo expanded mesenchymal stromal cell minimal quality requirements for clinical application. Stem Cells Dev. 2015;24(6):677–85. https://doi.org/10.1089/scd.2014.0299.
-
Mareschi K, Rustichelli D, Calabrese R, Gunetti M, Sanavio F, Castiglia S, et al. Multipotent mesenchymal stromal stem cell expansion by plating whole bone marrow at a low cellular density: a more advantageous method for clinical use. Stem Cells Int. 2012;2012: 920581. https://doi.org/10.1155/2012/920581.
-
Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115(8):1549–53. https://doi.org/10.1182/blood-2009-05-219907.
-
Castiglia S, Mareschi K, Labanca L, Lucania G, Leone M, Sanavio F, et al. Inactivated human platelet lysate with psoralen: a new perspective for mesenchymal stromal cell production in Good Manufacturing Practice conditions. Cytotherapy. 2014;16(6):750–63. https://doi.org/10.1016/j.jcyt.2013.12.008.
-
Zhang X, Kong Y, Sun Y, Qian Z, Gao C, Shi X, et al. Bone marrow mesenchymal stem cells conditioned medium protects VSC4.1 cells against 2,5-hexanedione-induced autophagy via NGF-PI3K/Akt/mTOR signaling pathway. Brain Res. 2018;1696:1–9. https://doi.org/10.1016/j.brainres.2018.04.028.
-
Rezaei N, Bojnordi MN, Ghasemi HH. Differentiation of bone marrow stromal stem cells seeded on silk scaffold to mature oligodendrocyte using cerebrospinal fluid. J Chem Neuroanat. 2020;106: 101790. https://doi.org/10.1016/j.jchemneu.2020.101790.
-
Cheng MT, Liu CL, Chen TH, Lee OK. Optimization of culture conditions for stem cells derived from human anterior cruciate ligament and bone marrow. Cell Transplant. 2014;23(7):791–803. https://doi.org/10.3727/096368912×666430.
-
Meuleman N, Tondreau T, Delforge A, Dejeneffe M, Massy M, Libertalis M, et al. Human marrow mesenchymal stem cell culture: serum-free medium allows better expansion than classical alpha-MEM medium. Eur J Haematol. 2006;76(4):309–16. https://doi.org/10.1111/j.1600-0609.2005.00611.x.
-
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95. https://doi.org/10.1091/mbc.e02-02-0105.
-
Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15(6):641–8. https://doi.org/10.1016/j.jcyt.2013.02.006.
-
Yoshimura K, Suga H, Eto H. Adipose-derived stem/progenitor cells: roles in adipose tissue remodeling and potential use for soft tissue augmentation. Regen Med. 2009;4(2):265–73. https://doi.org/10.2217/17460751.4.2.265.
-
Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–17. https://doi.org/10.1111/j.1749-6632.2009.04607.x.
-
Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301. https://doi.org/10.1634/stemcells.2005-0342.
-
Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99(5):1285–97. https://doi.org/10.1002/jcb.20904.
-
De Francesco F, Ricci G, D’Andrea F, Nicoletti GF, Ferraro GA. Human Adipose Stem Cells: From Bench to Bedside. Tissue Eng Part B Rev. 2015;21(6):572–84. https://doi.org/10.1089/ten.TEB.2014.0608.
-
Kd M, Ai I, Kt E, Wh M. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem Soc Trans. 1991;19(1):29S. https://doi.org/10.1042/bst019029s.
-
Chetty S, Yarani R, Swaminathan G, Primavera R, Regmi S, Rai S, et al. Umbilical cord mesenchymal stromal cells-from bench to bedside. Front Cell Dev Biol. 2022;10:1006295. https://doi.org/10.3389/fcell.2022.1006295.
-
Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34(3):695–704. https://doi.org/10.3892/ijmm.2014.1821.
-
Chen Y, Shen H, Ding Y, Yu Y, Shao L, Shen Z. The application of umbilical cord-derived MSCs in cardiovascular diseases. J Cell Mol Med. 2021;25(17):8103–14. https://doi.org/10.1111/jcmm.16830.
-
Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev Rep. 2013;9(2):226–40. https://doi.org/10.1007/s12015-012-9418-z.
-
Gao S, Jin Y, Ma J, Wang J, Wang J, Shao Z, et al. Preclinical study of human umbilical cord mesenchymal stem cell sheets for the recovery of ischemic heart tissue. Stem Cell Res Ther. 2022;13(1):252. https://doi.org/10.1186/s13287-022-02919-8.
-
Margossian T, Reppel L, Makdissy N, Stoltz JF, Bensoussan D, Huselstein C. Mesenchymal stem cells derived from Wharton’s jelly: comparative phenotype analysis between tissue and in vitro expansion. Biomed Mater Eng. 2012;22(4):243–54. https://doi.org/10.3233/bme-2012-0714.
-
Yoon JH, Roh EY, Shin S, Jung NH, Song EY, Chang JY, et al. Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton’s jelly. Biomed Res Int. 2013;2013: 428726. https://doi.org/10.1155/2013/428726.
-
Semenova E, Grudniak MP, Machaj EK, Bocian K, Chroscinska-Krawczyk M, Trochonowicz M, et al. Mesenchymal Stromal Cells from Different Parts of Umbilical Cord: Approach to Comparison & Characteristics. Stem Cell Rev Rep. 2021;17(5):1780–95. https://doi.org/10.1007/s12015-021-10157-3.
-
Feng X, Liu J, Xu Y, Zhu J, Chen W, Feng B, et al. Molecular mechanism underlying the difference in proliferation between placenta-derived and umbilical cord-derived mesenchymal stem cells. J Cell Physiol. 2020;235(10):6779–93. https://doi.org/10.1002/jcp.29572.
-
Chia WK, Cheah FC, Abdul Aziz NH, Kampan NC, Shuib S, Khong TY, et al. A Review of Placenta and Umbilical Cord-Derived Stem Cells and the Immunomodulatory Basis of Their Therapeutic Potential in Bronchopulmonary Dysplasia. Front Pediatr. 2021;9: 615508. https://doi.org/10.3389/fped.2021.615508.
-
Aramburú Junior JS, Eilers Treichel TL, Lemos Pinto Filho ST, Gehrke SA, Machado AK, Cadoná FC, et al. DNA damage in dental pulp mesenchymal stem cells: An in vitro study. Vet Res Forum. 2018;9(4):293–9. https://doi.org/10.30466/vrf.2018.33083.
-
Stanko P, Altanerova U, Jakubechova J, Repiska V, Altaner C. Dental Mesenchymal Stem/Stromal Cells and Their Exosomes. Stem Cells Int. 2018;2018:8973613. https://doi.org/10.1155/2018/8973613.
-
Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M. Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod. 2009;35(11):1536–42. https://doi.org/10.1016/j.joen.2009.07.024.
-
Lee YC, Chan YH, Hsieh SC, Lew WZ, Feng SW. Comparing the Osteogenic Potentials and Bone Regeneration Capacities of Bone Marrow and Dental Pulp Mesenchymal Stem Cells in a Rabbit Calvarial Bone Defect Model. Int J Mol Sci. 2019;20(20). https://doi.org/10.3390/ijms20205015.
-
M D, JC F, M P, E P-G, A M, F M-G, et al. Phenotypic Identification of Dental Pulp Mesenchymal Stem/Stromal Cells Subpopulations with Multiparametric Flow Cytometry. Methods in molecular biology (Clifton, NJ). 2019;1922:77–90. https://doi.org/10.1007/978-1-4939-9012-2_8.
-
KB G, DS S, AF E, DN S, AC dA, RR DS, et al. Conditioned Medium of Bone Marrow-Derived Mesenchymal Stromal Cells as a Therapeutic Approach to Neuropathic Pain: A Preclinical Evaluation. Stem cells international. 2018;2018:8179013. https://doi.org/10.1155/2018/8179013.
-
Sekiya I, Muneta T, Horie M, Koga H. Arthroscopic Transplantation of Synovial Stem Cells Improves Clinical Outcomes in Knees With Cartilage Defects. Clin Orthop Relat Res. 2015;473(7):2316–26. https://doi.org/10.1007/s11999-015-4324-8.
-
Guo L, Qin W, Cai Z, Su X. Hybrid Clustering Algorithm Based on Improved Density Peak Clustering. Appl Sci. 2024;14(2):715.
-
N K, S N, M Y, T O. Properties of Dental Pulp-derived Mesenchymal Stem Cells and the Effects of Culture Conditions. Journal of endodontics. 2017;43(9S):S31-S4. https://doi.org/10.1016/j.joen.2017.06.004.
-
Noda S, Kawashima N, Yamamoto M, Hashimoto K, Nara K, Sekiya I, et al. Effect of cell culture density on dental pulp-derived mesenchymal stem cells with reference to osteogenic differentiation. Sci Rep. 2019;9(1):5430. https://doi.org/10.1038/s41598-019-41741-w.
-
Shen WC, Lai YC, Li LH, Liao K, Lai HC, Kao SY, et al. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat Commun. 2019;10(1):2226. https://doi.org/10.1038/s41467-019-10197-x.
-
Eiro N, Fraile M, Schneider J, Vizoso FJ. Non Pregnant Human Uterus as Source of Mesenchymal Stem Cells. Curr Stem Cell Res Ther. 2018;13(6):423–31. https://doi.org/10.2174/1381612824666180426120459.
-
Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. https://doi.org/10.1186/1478-811x-9-12.
-
Nili E, Li FJ, Dawson RA, Lau C, McEwan B, Barnett NL, et al. The Impact of Limbal Mesenchymal Stromal Cells on Healing of Acute Ocular Surface Wounds Is Improved by Pre-cultivation and Implantation in the Presence of Limbal Epithelial Cells. Cell Transplant. 2019;28(9–10):1257–70. https://doi.org/10.1177/0963689719858577.
-
Duan W, Wang H, Wang Z, Ren Z, Li X, He F, et al. Multi-functional composite dressings with sustained release of MSC-SLP and anti-adhesion property for accelerating wound healing. Mater Today Bio. 2024;25: 100979. https://doi.org/10.1016/j.mtbio.2024.100979.
-
Costela-Ruiz VJ, Melguizo-Rodríguez L, Bellotti C, Illescas-Montes R, Stanco D, Arciola CR, et al. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int J Mol Sci. 2022;23(11). https://doi.org/10.3390/ijms23116356.
-
Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Péault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21(8):1299–308. https://doi.org/10.1089/scd.2011.0200.
-
Garikipati VNS, Singh SP, Mohanram Y, Gupta AK, Kapoor D, Nityanand S. Isolation and characterization of mesenchymal stem cells from human fetus heart. PLoS ONE. 2018;13(2): e0192244. https://doi.org/10.1371/journal.pone.0192244.
-
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. https://doi.org/10.1080/20013078.2018.1535750.
-
He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: Biology and Translational Medicine. Theranostics. 2018;8(1):237–55. https://doi.org/10.7150/thno.21945.
-
Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, et al. Progress, opportunity, and perspective on exosome isolation – efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–707. https://doi.org/10.7150/thno.41580.
-
Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. https://doi.org/10.1002/0471143030.cb0322s30.
-
Ansari FJ, Tafti HA, Amanzadeh A, Rabbani S, Shokrgozar MA, Heidari R, et al. Comparison of the efficiency of ultrafiltration, precipitation, and ultracentrifugation methods for exosome isolation. Biochem Biophys Rep. 2024;38: 101668. https://doi.org/10.1016/j.bbrep.2024.101668.
-
Soares Martins T, Catita J, Martins Rosa I, O ABdCES, Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS One. 2018;13(6):e0198820. https://doi.org/10.1371/journal.pone.0198820.
-
Hartjes TA, Mytnyk S, Jenster GW, van Steijn V, van Royen ME. Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering (Basel). 2019;6(1). https://doi.org/10.3390/bioengineering6010007.
-
Rohde E, Pachler K, Gimona M. Manufacturing and characterization of extracellular vesicles from umbilical cord-derived mesenchymal stromal cells for clinical testing. Cytotherapy. 2019;21(6):581–92. https://doi.org/10.1016/j.jcyt.2018.12.006.
-
Stawarska A, Bamburowicz-Klimkowska M, Runden-Pran E, Dusinska M, Cimpan MR, Rios-Mondragon I, et al. Extracellular Vesicles as Next-Generation Diagnostics and Advanced Therapy Medicinal Products. Int J Mol Sci. 2024;25(12). https://doi.org/10.3390/ijms25126533.
-
Wang CK, Tsai TH, Lee CH. Regulation of exosomes as biologic medicines: Regulatory challenges faced in exosome development and manufacturing processes. Clin Transl Sci. 2024;17(8): e13904. https://doi.org/10.1111/cts.13904.
-
Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75(2):193–208. https://doi.org/10.1007/s00018-017-2595-9.
-
Ala M. The beneficial effects of mesenchymal stem cells and their exosomes on myocardial infarction and critical considerations for enhancing their efficacy. Ageing Res Rev. 2023;89: 101980. https://doi.org/10.1016/j.arr.2023.101980.
-
Shetgaonkar GG, Marques SM, CEM DC, Vibhavari RJA, Kumar L, Shirodkar RK. Exosomes as cell-derivative carriers in the diagnosis and treatment of central nervous system diseases. Drug Deliv Transl Res. 2022;12(5):1047–79. https://doi.org/10.1007/s13346-021-01026-0.
-
Yu X, Odenthal M, Fries JW. Exosomes as miRNA Carriers: Formation-Function-Future. Int J Mol Sci. 2016;17(12). https://doi.org/10.3390/ijms17122028.
-
Sheng ZH, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13(2):77–93. https://doi.org/10.1038/nrn3156.
-
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535(7613):551–5. https://doi.org/10.1038/nature18928.
-
Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med. 2017;196(10):1275–86. https://doi.org/10.1164/rccm.201701-0170OC.
-
Qin Y, Jiang X, Yang Q, Zhao J, Zhou Q, Zhou Y. The Functions, Methods, and Mobility of Mitochondrial Transfer Between Cells. Front Oncol. 2021;11: 672781. https://doi.org/10.3389/fonc.2021.672781.
-
Yang J, Liu L, Oda Y, Wada K, Ago M, Matsuda S, et al. Extracellular Vesicles and Cx43-Gap Junction Channels Are the Main Routes for Mitochondrial Transfer from Ultra-Purified Mesenchymal Stem Cells, RECs. Int J Mol Sci. 2023;24(12). https://doi.org/10.3390/ijms241210294.
-
Horibe S, Tanahashi T, Kawauchi S, Murakami Y, Rikitake Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer. 2018;18(1):47. https://doi.org/10.1186/s12885-017-3958-1.
-
Willms E, Cabañas C, Mäger I, Wood MJA, Vader P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front Immunol. 2018;9:738. https://doi.org/10.3389/fimmu.2018.00738.
-
Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–35. https://doi.org/10.1038/nature15756.
-
Sorgen PL, Trease AJ, Spagnol G, Delmar M, Nielsen MS. Protein⁻Protein Interactions with Connexin 43: Regulation and Function. International journal of molecular sciences. 2018;19(5). https://doi.org/10.3390/ijms19051428.
-
Shimaoka M, Kawamoto E, Gaowa A, Okamoto T, Park EJ. Connexins and Integrins in Exosomes. Cancers. 2019;11(1). https://doi.org/10.3390/cancers11010106.
-
Mathieu M, Névo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12(1):4389. https://doi.org/10.1038/s41467-021-24384-2.
-
Fan Y, Pionneau C, Cocozza F, Boëlle PY, Chardonnet S, Charrin S, et al. Differential proteomics argues against a general role for CD9, CD81 or CD63 in the sorting of proteins into extracellular vesicles. J Extracell Vesicles. 2023;12(8): e12352. https://doi.org/10.1002/jev2.12352.
-
Ai Y, Guo C, Garcia-Contreras M, Sánchez BL, Saftics A, Shodubi O, et al. Endocytosis blocks the vesicular secretion of exosome marker proteins. Sci Adv. 2024;10(19):eadi9156. https://doi.org/10.1126/sciadv.adi9156.
-
Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. https://doi.org/10.3402/jev.v4.26316.
-
Watson DC, Bayik D, Srivatsan A, Bergamaschi C, Valentin A, Niu G, et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials. 2016;105:195–205. https://doi.org/10.1016/j.biomaterials.2016.07.003.
-
Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. 2024;9(1):17. https://doi.org/10.1038/s41392-023-01704-0.
-
T LR, Sánchez-Abarca LI, Muntión S, Preciado S, Puig N, López-Ruano G, et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun Signal. 2016;14:2. https://doi.org/10.1186/s12964-015-0124-8.
-
Wang S, Li L, Liu T, Jiang W, Hu X. miR-19a/19b-loaded exosomes in combination with mesenchymal stem cell transplantation in a preclinical model of myocardial infarction. Regen Med. 2020;15(6):1749–59. https://doi.org/10.2217/rme-2019-0136.
-
Wang L, Jia Q, Xinnong C, Xie Y, Yang Y, Zhang A, et al. Role of cardiac progenitor cell-derived exosome-mediated microRNA-210 in cardiovascular disease. J Cell Mol Med. 2019;23(11):7124–31. https://doi.org/10.1111/jcmm.14562.
-
Pan Q, Kuang X, Cai S, Wang X, Du D, Wang J, et al. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res Ther. 2020;11(1):260. https://doi.org/10.1186/s13287-020-01761-0.
-
Zhai K, Deng L, Wu Y, Li H, Zhou J, Shi Y, et al. Extracellular vesicle-derived miR-146a as a novel crosstalk mechanism for high-fat induced atherosclerosis by targeting SMAD4. J Adv Res. 2024. https://doi.org/10.1016/j.jare.2024.08.012.
-
Poulet C, Njock MS, Moermans C, Louis E, Louis R, Malaise M, et al. Exosomal Long Non-Coding RNAs in Lung Diseases. International journal of molecular sciences. 2020;21(10). https://doi.org/10.3390/ijms21103580.
-
Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from hypoxia-treated human adipose-derived mesenchymal stem cells enhance angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59–68. https://doi.org/10.1016/j.biocel.2019.01.017.
-
Divband S, Tasharrofi N, Abroun S, Soufi ZM. Human Umbilical Cord Mesenchymal Stem Cells-Derived Small Extracellular Vesicles Can Be Considered as Cell-Free Therapeutics for Angiogenesis Promotion. Cell J. 2022;24(11):689–96. https://doi.org/10.22074/cellj.2022.8275.
-
Zhou H, Wu Y, Xue J, Yu L. Ameliorative effects of HGF-overexpressed exosomes derived from ADMSCs on oxidative stress in hepatic fibrosis. Histology and histopathology. 2024:18816. https://doi.org/10.14670/hh-18-816.
-
Liu Y, Wang M, Yu Y, Li C, Zhang C. Advances in the study of exosomes derived from mesenchymal stem cells and cardiac cells for the treatment of myocardial infarction. Cell Commun Signal. 2023;21(1):202. https://doi.org/10.1186/s12964-023-01227-9.
-
Wang ZG, He ZY, Liang S, Yang Q, Cheng P, Chen AM. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):511. https://doi.org/10.1186/s13287-020-02032-8.
-
Li Q, Xu Y, Lv K, Wang Y, Zhong Z, Xiao C, et al. Small extracellular vesicles containing miR-486–5p promote angiogenesis after myocardial infarction in mice and nonhuman primates. Sci Transl Med. 2021;13(584). https://doi.org/10.1126/scitranslmed.abb0202.
-
Shao M, Xu Q, Wu Z, Chen Y, Shu Y, Cao X, et al. Exosomes derived from human umbilical cord mesenchymal stem cells ameliorate IL-6-induced acute liver injury through miR-455-3p. Stem Cell Res Ther. 2020;11(1):37. https://doi.org/10.1186/s13287-020-1550-0.
-
Dong L, Pu Y, Zhang L, Qi Q, Xu L, Li W, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018;9(2):218. https://doi.org/10.1038/s41419-018-0323-5.
-
Yoo JK, Kim CH, Jung HY, Lee DR, Kim JK. Discovery and characterization of miRNA during cellular senescence in bone marrow-derived human mesenchymal stem cells. Exp Gerontol. 2014;58:139–45. https://doi.org/10.1016/j.exger.2014.07.020.
-
Lu GM, Rong YX, Liang ZJ, Hunag DL, Ma YF, Luo ZZ, et al. Multiomics global landscape of stemness-related gene clusters in adipose-derived mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):310. https://doi.org/10.1186/s13287-020-01823-3.
-
Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017;26(9):617–31. https://doi.org/10.1089/scd.2016.0349.
-
Pincela Lins PM, Pirlet E, Szymonik M, Bronckaers A, Nelissen I. Manufacture of extracellular vesicles derived from mesenchymal stromal cells. Trends Biotechnol. 2023;41(7):965–81. https://doi.org/10.1016/j.tibtech.2023.01.003.
-
L PK, Kandoi S, Misra R, S V, K R, Verma RS. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1–9. https://doi.org/10.1016/j.cytogfr.2019.04.002.
-
Chang C, Yan J, Yao Z, Zhang C, Li X, Mao HQ. Effects of Mesenchymal Stem Cell-Derived Paracrine Signals and Their Delivery Strategies. Adv Healthc Mater. 2021;10(7): e2001689. https://doi.org/10.1002/adhm.202001689.
-
Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, et al. The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduct Target Ther. 2022;7(1):92. https://doi.org/10.1038/s41392-022-00932-0.
-
Yamaguchi S, Shibata R, Yamamoto N, Nishikawa M, Hibi H, Tanigawa T, et al. Dental pulp-derived stem cell conditioned medium reduces cardiac injury following ischemia-reperfusion. Sci Rep. 2015;5:16295. https://doi.org/10.1038/srep16295.
-
Lee TL, Lai TC, Lin SR, Lin SW, Chen YC, Pu CM, et al. Conditioned medium from adipose-derived stem cells attenuates ischemia/reperfusion-induced cardiac injury through the microRNA-221/222/PUMA/ETS-1 pathway. Theranostics. 2021;11(7):3131–49. https://doi.org/10.7150/thno.52677.
-
Baldassarro VA, Perut F, Cescatti M, Pinto V, Fazio N, Alastra G, et al. Intra-individual variability in the neuroprotective and promyelinating properties of conditioned culture medium obtained from human adipose mesenchymal stromal cells. Stem Cell Res Ther. 2023;14(1):128. https://doi.org/10.1186/s13287-023-03344-1.
-
Yang Y, Lee EH, Yang Z. Hypoxia-Conditioned Mesenchymal Stem Cells in Tissue Regeneration Application. Tissue Eng Part B Rev. 2022;28(5):966–77. https://doi.org/10.1089/ten.TEB.2021.0145.
-
Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284(48):33161–8. https://doi.org/10.1074/jbc.M109.020925.
-
Hwang N, Ghanta S, Li Q, Lamattina AM, Murzin E, Lederer JA, et al. Carbon monoxide-induced autophagy enhances human mesenchymal stromal cell function via paracrine actions in murine polymicrobial sepsis. Mol Ther. 2024;32(7):2232–47. https://doi.org/10.1016/j.ymthe.2024.05.018.
-
Parate D, Kadir ND, Celik C, Lee EH, Hui JHP, Franco-Obregón A, et al. Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Stem Cell Res Ther. 2020;11(1):46. https://doi.org/10.1186/s13287-020-1566-5.
-
Heo JS. Selenium-Stimulated Exosomes Enhance Wound Healing by Modulating Inflammation and Angiogenesis. International journal of molecular sciences. 2022;23(19). https://doi.org/10.3390/ijms231911543.
-
Liu X, Duan B, Cheng Z, Jia X, Mao L, Fu H, et al. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011;2(10):845–54. https://doi.org/10.1007/s13238-011-1097-z.
-
Ariyanti AD, Zhang J, Marcelina O, Nugrahaningrum DA, Wang G, Kasim V, et al. Salidroside-Pretreated Mesenchymal Stem Cells Enhance Diabetic Wound Healing by Promoting Paracrine Function and Survival of Mesenchymal Stem Cells Under Hyperglycemia. Stem Cells Transl Med. 2019;8(4):404–14. https://doi.org/10.1002/sctm.18-0143.
-
Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE. 2014;9(2): e88685. https://doi.org/10.1371/journal.pone.0088685.
-
Sánchez-Sánchez R, Gómez-Ferrer M, Reinal I, Buigues M, Villanueva-Bádenas E, Ontoria-Oviedo I, et al. miR-4732-3p in Extracellular Vesicles From Mesenchymal Stromal Cells Is Cardioprotective During Myocardial Ischemia. Front Cell Dev Biol. 2021;9: 734143. https://doi.org/10.3389/fcell.2021.734143.
-
Santos Nascimento D, Mosqueira D, Sousa LM, Teixeira M, Filipe M, Resende TP, et al. Human umbilical cord tissue-derived mesenchymal stromal cells attenuate remodeling after myocardial infarction by proangiogenic, antiapoptotic, and endogenous cell-activation mechanisms. Stem Cell Res Ther. 2014;5(1):5. https://doi.org/10.1186/scrt394.
-
Yang Y, Wu Y, Yang D, Neo SH, Kadir ND, Goh D, et al. Secretive derived from hypoxia preconditioned mesenchymal stem cells promote cartilage regeneration and mitigate joint inflammation via extracellular vesicles. Bioact Mater. 2023;27:98–112. https://doi.org/10.1016/j.bioactmat.2023.03.017.
-
Kim H, Yu MR, Lee H, Kwon SH, Jeon JS, Han DC, et al. Metformin inhibits chronic kidney disease-induced DNA damage and senescence of mesenchymal stem cells. Aging Cell. 2021;20(2): e13317. https://doi.org/10.1111/acel.13317.
-
Sun J, Shen H, Shao L, Teng X, Chen Y, Liu X, et al. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther. 2020;11(1):373. https://doi.org/10.1186/s13287-020-01881-7.
-
Tang J, Wang J, Yang J, Kong X, Zheng F, Guo L, et al. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36(4):644–50. https://doi.org/10.1016/j.ejcts.2009.04.052.
-
Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46(7):1339–50. https://doi.org/10.1016/j.jacc.2005.05.079.
-
Wu SZ, Li YL, Huang W, Cai WF, Liang J, Paul C, et al. Paracrine effect of CXCR4-overexpressing mesenchymal stem cells on ischemic heart injury. Cell Biochem Funct. 2017;35(2):113–23. https://doi.org/10.1002/cbf.3254.
-
Yang M, Liao M, Liu R, Zhang Q, Zhang S, He Y, et al. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles loaded with miR-223 ameliorate myocardial infarction through P53/S100A9 axis. Genomics. 2022;114(3): 110319. https://doi.org/10.1016/j.ygeno.2022.110319.
-
Chen JJ, Zhou SH. Mesenchymal stem cells overexpressing MiR-126 enhance ischemic angiogenesis via the AKT/ERK-related pathway. Cardiol J. 2011;18(6):675–81. https://doi.org/10.5603/cj.2011.0032.
-
Huang F, Zhu X, Hu XQ, Fang ZF, Tang L, Lu XL, et al. Mesenchymal stem cells modified with miR-126 release angiogenic factors and activate Notch ligand Delta-like-4, enhancing ischemic angiogenesis and cell survival. Int J Mol Med. 2013;31(2):484–92. https://doi.org/10.3892/ijmm.2012.1200.
-
Li Y, Huang J, Wang J, Xia S, Ran H, Gao L, et al. Human umbilical cord-derived mesenchymal stem cell transplantation supplemented with curcumin improves the outcomes of ischemic stroke via AKT/GSK-3β/β-TrCP/Nrf2 axis. J Neuroinflammation. 2023;20(1):49. https://doi.org/10.1186/s12974-023-02738-5.
-
Cheng S, Lu Q, Liu Q, Ma Y, Chen J, Lu D, et al. Synergistic effects of repeated transcranial magnetic stimulation and mesenchymal stem cells transplantation on alleviating neuroinflammation and PANoptosis in cerebral ischemia. J Neuroinflammation. 2024;21(1):311. https://doi.org/10.1186/s12974-024-03302-5.
-
Wang Y, Chen L, Wang L, Pei G, Cheng H, Zhang Q, et al. Pulsed Electromagnetic Fields Combined With Adipose-Derived Stem Cells Protect Ischemic Myocardium by Regulating miR-20a-5p/E2F1/p73 Signaling. Stem cells (Dayton, Ohio). 2023;41(7):724–37. https://doi.org/10.1093/stmcls/sxad037.
-
Li Z, Yang B, Yang Z, Xie X, Guo Z, Zhao J, et al. Supramolecular Hydrogel with Ultra-Rapid Cell-Mediated Network Adaptation for Enhancing Cellular Metabolic Energetics and Tissue Regeneration. Advanced materials (Deerfield Beach, Fla). 2024;36(15): e2307176. https://doi.org/10.1002/adma.202307176.
-
Chen Y, Shu Z, Qian K, Wang J, Zhu H. Harnessing the Properties of Biomaterial to Enhance the Immunomodulation of Mesenchymal Stem Cells. Tissue Eng Part B Rev. 2019;25(6):492–9. https://doi.org/10.1089/ten.TEB.2019.0131.
-
Lee MC, Lee JS, Kim S, Jamaiyar A, Wu W, Gonzalez ML, et al. Synergistic effect of Hypoxic Conditioning and Cell-Tethering Colloidal Gels enhanced Productivity of MSC Paracrine Factors and Accelerated Vessel Regeneration. Advanced materials (Deerfield Beach, Fla). 2024:e2408488. https://doi.org/10.1002/adma.202408488.
-
Li X, Li X, Yang J, Lin J, Zhu Y, Xu X, et al. Living and Injectable Porous Hydrogel Microsphere with Paracrine Activity for Cartilage Regeneration. Small. 2023;19(17): e2207211. https://doi.org/10.1002/smll.202207211.
-
Sun L, Wang X, Saredy J, Yuan Z, Yang X, Wang H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020;37: 101759. https://doi.org/10.1016/j.redox.2020.101759.
-
Wu X, Reboll MR, Korf-Klingebiel M, Wollert KC. Angiogenesis after acute myocardial infarction. Cardiovasc Res. 2021;117(5):1257–73. https://doi.org/10.1093/cvr/cvaa287.
-
van den Akker F, Deddens JC, Doevendans PA, Sluijter JP. Cardiac stem cell therapy to modulate inflammation upon myocardial infarction. Biochim Biophys Acta. 2013;1830(2):2449–58. https://doi.org/10.1016/j.bbagen.2012.08.026.
-
Tang J, Chen Y, Wang C, Xia Y, Yu T, Tang M, et al. The role of mesenchymal stem cells in cancer and prospects for their use in cancer therapeutics. MedComm (2020). 2024;5(8):e663. https://doi.org/10.1002/mco2.663.
-
Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17(7):387–401. https://doi.org/10.1038/s41569-020-0352-5.
-
Sage AP, Tsiantoulas D, Binder CJ, Mallat Z. The role of B cells in atherosclerosis. Nat Rev Cardiol. 2019;16(3):180–96. https://doi.org/10.1038/s41569-018-0106-9.
-
McHugh J. T(reg) cell-inducing nanoparticles show promise for treating OA. Nat Rev Rheumatol. 2023;19(2):62. https://doi.org/10.1038/s41584-023-00906-8.
-
Sharabi A, Tsokos GC. T cell metabolism: new insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol. 2020;16(2):100–12. https://doi.org/10.1038/s41584-019-0356-x.
-
Vilahur G, Juan-Babot O, Peña E, Oñate B, Casaní L, Badimon L. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J Mol Cell Cardiol. 2011;50(3):522–33. https://doi.org/10.1016/j.yjmcc.2010.12.021.
-
Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97: 107823. https://doi.org/10.1016/j.intimp.2021.107823.
-
Copp G, Robb KP, Viswanathan S. Culture-expanded mesenchymal stromal cell therapy: does it work in knee osteoarthritis? A pathway to clinical success. Cell Mol Immunol. 2023;20(6):626–50. https://doi.org/10.1038/s41423-023-01020-1.
-
Court AC, Vega-Letter AM, Parra-Crisóstomo E, Velarde F, García C, Ortloff A, et al. Mitochondrial transfer balances cell redox, energy and metabolic homeostasis in the osteoarthritic chondrocyte preserving cartilage integrity. Theranostics. 2024;14(17):6471–86. https://doi.org/10.7150/thno.96723.
-
Peng S, Sun C, Lai C, Zhang L. Exosomes derived from mesenchymal stem cells rescue cartilage injury in osteoarthritis through Ferroptosis by GOT1/CCR2 expression. Int Immunopharmacol. 2023;122: 110566. https://doi.org/10.1016/j.intimp.2023.110566.
-
Lagneau N, Tournier P, Nativel F, Maugars Y, Guicheux J, Le Visage C, et al. Harnessing cell-material interactions to control stem cell secretion for osteoarthritis treatment. Biomaterials. 2023;296: 122091. https://doi.org/10.1016/j.biomaterials.2023.122091.
-
Pang L, Jin H, Lu Z, Xie F, Shen H, Li X, et al. Treatment with Mesenchymal Stem Cell-Derived Nanovesicle-Containing Gelatin Methacryloyl Hydrogels Alleviates Osteoarthritis by Modulating Chondrogenesis and Macrophage Polarization. Adv Healthc Mater. 2023;12(17): e2300315. https://doi.org/10.1002/adhm.202300315.
-
Colonna M. TREM1 Blockade: Killing Two Birds with One Stone. Trends Immunol. 2019;40(9):781–3. https://doi.org/10.1016/j.it.2019.07.008.
-
Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16(1):178. https://doi.org/10.1186/s12974-019-1571-8.
-
Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9(1):42. https://doi.org/10.1186/s40035-020-00221-2.
-
Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154(2):204–19. https://doi.org/10.1111/imm.12922.
-
Chen Y, Li J, Ma B, Li N, Wang S, Sun Z, et al. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging. 2020;12(18):18274–96. https://doi.org/10.18632/aging.103692.
-
Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, et al. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17(1):47. https://doi.org/10.1186/s12974-020-1726-7.
-
Kang J, Guo Y. Human Umbilical Cord Mesenchymal Stem Cells Derived Exosomes Promote Neurological Function Recovery in a Rat Spinal Cord Injury Model. Neurochem Res. 2022;47(6):1532–40. https://doi.org/10.1007/s11064-022-03545-9.
-
Yang Y, Liu Q, Deng S, Shao Q, Peng L, Ling Y, et al. Human umbilical cord derived mesenchymal stem cells overexpressing HO-1 attenuate neural injury and enhance functional recovery by inhibiting inflammation in stroke mice. CNS Neurosci Ther. 2024;30(2): e14412. https://doi.org/10.1111/cns.14412.
-
Matusiewicz A, Stróżyńska-Byrska J, Olesińska M. Polyautoimmunity in rheumatological conditions. Int J Rheum Dis. 2019;22(3):386–91. https://doi.org/10.1111/1756-185x.13454.
-
Porsch F, Binder CJ. Autoimmune diseases and atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2024;21(11):780–807. https://doi.org/10.1038/s41569-024-01045-7.
-
Zaripova LN, Midgley A, Christmas SE, Beresford MW, Pain C, Baildam EM, et al. Mesenchymal Stem Cells in the Pathogenesis and Therapy of Autoimmune and Autoinflammatory Diseases. International journal of molecular sciences. 2023;24(22). https://doi.org/10.3390/ijms242216040.
-
Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41(3):283–97. https://doi.org/10.1007/s00281-019-00733-8.
-
Park JS, Perl A. Endosome Traffic Modulates Pro-Inflammatory Signal Transduction in CD4(+) T Cells-Implications for the Pathogenesis of Systemic Lupus Erythematosus. International journal of molecular sciences. 2023;24(13). https://doi.org/10.3390/ijms241310749.
-
Ding S, Ren T, Song S, Peng C, Liu C, Wu J, et al. Combined application of mesenchymal stem cells and different glucocorticoid dosing alleviates osteoporosis in SLE murine models. Immun Inflamm Dis. 2024;12(6): e1319. https://doi.org/10.1002/iid3.1319.
-
Ma W, Che J, Chen W, Wang D, Zhang H, Zhao Y. Dexamethasone-Integrated Mesenchymal Stem Cells for Systemic Lupus Erythematosus Treatment via Multiple Immunomodulatory Mechanisms. ACS Nano. 2024;18(20):13249–65. https://doi.org/10.1021/acsnano.4c02420.
-
Ma L, Wu H, Cao J, Zhang N, Li Y, Zheng J, et al. Mesenchymal Stem Cell-Based Biomimetic Liposome for Targeted Treatment of Rheumatoid Arthritis. ACS Appl Mater Interfaces. 2024;16(36):47206–15. https://doi.org/10.1021/acsami.4c09080.
-
Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30(3–4):97–104. https://doi.org/10.1007/s10753-007-9025-3.
-
Liu Y, Guan R, Yan J, Zhu Y, Sun S, Qu Y. Mesenchymal Stem Cell-Derived Extracellular Vesicle-Shuttled microRNA-302d-3p Represses Inflammation and Cardiac Remodeling Following Acute Myocardial Infarction. J Cardiovasc Transl Res. 2022;15(4):754–71. https://doi.org/10.1007/s12265-021-10200-1.
-
Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, et al. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res. 2019;115(7):1205–16. https://doi.org/10.1093/cvr/cvz040.
-
Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019;8(5). https://doi.org/10.3390/cells8050467.
-
Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, et al. miR-93-5p-Containing Exosomes Treatment Attenuates Acute Myocardial Infarction-Induced Myocardial Damage. Mol Ther Nucleic Acids. 2018;11:103–15. https://doi.org/10.1016/j.omtn.2018.01.010.
-
Wei Z, Qiao S, Zhao J, Liu Y, Li Q, Wei Z, et al. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci. 2019;232: 116632. https://doi.org/10.1016/j.lfs.2019.116632.
-
Cassatella MA, Mosna F, Micheletti A, Lisi V, Tamassia N, Cont C, et al. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011;29(6):1001–11. https://doi.org/10.1002/stem.651.
-
Jiang D, Muschhammer J, Qi Y, Kügler A, de Vries JC, Saffarzadeh M, et al. Suppression of Neutrophil-Mediated Tissue Damage-A Novel Skill of Mesenchymal Stem Cells. Stem Cells. 2016;34(9):2393–406. https://doi.org/10.1002/stem.2417.
-
Loh JT, Zhang B, Teo JKH, Lai RC, Choo ABH, Lam KP, et al. Mechanism for the attenuation of neutrophil and complement hyperactivity by MSC exosomes. Cytotherapy. 2022;24(7):711–9. https://doi.org/10.1016/j.jcyt.2021.12.003.
-
Su VY, Lin CS, Hung SC, Yang KY. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury. Int J Mol Sci. 2019;20(9). https://doi.org/10.3390/ijms20092208.
-
Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46(1): e70. https://doi.org/10.1038/emm.2013.135.
-
Chen Y, Zuo J, Chen W, Yang Z, Zhang Y, Hua F, et al. The enhanced effect and underlying mechanisms of mesenchymal stem cells with IL-33 overexpression on myocardial infarction. Stem Cell Res Ther. 2019;10(1):295. https://doi.org/10.1186/s13287-019-1392-9.
-
Wang J, Lee CJ, Deci MB, Jasiewicz N, Verma A, Canty JM, et al. MiR-101a loaded extracellular nanovesicles as bioactive carriers for cardiac repair. Nanomedicine. 2020;27: 102201. https://doi.org/10.1016/j.nano.2020.102201.
-
Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6(11):1028–42. https://doi.org/10.1038/sj.cdd.4400598.
-
Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai). 2005;37(11):719–27. https://doi.org/10.1111/j.1745-7270.2005.00108.x.
-
Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9(3):459–70. https://doi.org/10.1016/s1097-2765(02)00482-3.
-
Mocanu MM, Baxter GF, Yellon DM. Caspase inhibition and limitation of myocardial infarct size: protection against lethal reperfusion injury. Br J Pharmacol. 2000;130(2):197–200. https://doi.org/10.1038/sj.bjp.0703336.
-
Schwarz K, Simonis G, Yu X, Wiedemann S, Strasser RH. Apoptosis at a distance: remote activation of caspase-3 occurs early after myocardial infarction. Mol Cell Biochem. 2006;281(1–2):45–54. https://doi.org/10.1007/s11010-006-0233-1.
-
Chandrashekhar Y, Sen S, Anway R, Shuros A, Anand I. Long-term caspase inhibition ameliorates apoptosis, reduces myocardial troponin-I cleavage, protects left ventricular function, and attenuates remodeling in rats with myocardial infarction. J Am Coll Cardiol. 2004;43(2):295–301. https://doi.org/10.1016/j.jacc.2003.09.026.
-
Liu Q. Lentivirus mediated interference of Caspase-3 expression ameliorates the heart function on rats with acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2014;18(13):1852–8.
-
Gao L, Bledsoe G, Yin H, Shen B, Chao L, Chao J. Tissue kallikrein-modified mesenchymal stem cells provide enhanced protection against ischemic cardiac injury after myocardial infarction. Circ J. 2013;77(8):2134–44. https://doi.org/10.1253/circj.cj-12-1585.
-
Ham O, Lee SY, Lee CY, Park JH, Lee J, Seo HH, et al. let-7b suppresses apoptosis and autophagy of human mesenchymal stem cells transplanted into ischemia/reperfusion injured heart 7by targeting caspase-3. Stem Cell Res Ther. 2015;6(1):147. https://doi.org/10.1186/s13287-015-0134-x.
-
Liu X, Li X, Zhu W, Zhang Y, Hong Y, Liang X, et al. Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. J Cell Physiol. 2020;235(11):8010–22. https://doi.org/10.1002/jcp.29456.
-
Xu H, Wang Z, Liu L, Zhang B, Li B. Exosomes derived from adipose tissue, bone marrow, and umbilical cord blood for cardioprotection after myocardial infarction. J Cell Biochem. 2020;121(3):2089–102. https://doi.org/10.1002/jcb.27399.
-
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10(3):301–12. https://doi.org/10.1016/j.scr.2013.01.002.
-
Wang X, Chen Y, Zhao Z, Meng Q, Yu Y, Sun J, et al. Engineered Exosomes With Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J Am Heart Assoc. 2018;7(15): e008737. https://doi.org/10.1161/jaha.118.008737.
-
Mao Q, Liang XL, Zhang CL, Pang YH, Lu YX. LncRNA KLF3-AS1 in human mesenchymal stem cell-derived exosomes ameliorates pyroptosis of cardiomyocytes and myocardial infarction through miR-138-5p/Sirt1 axis. Stem Cell Res Ther. 2019;10(1):393. https://doi.org/10.1186/s13287-019-1522-4.
-
Luther KM, Haar L, McGuinness M, Wang Y, Lynch Iv TL, Phan A, et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J Mol Cell Cardiol. 2018;119:125–37. https://doi.org/10.1016/j.yjmcc.2018.04.012.
-
Cheng H, Chang S, Xu R, Chen L, Song X, Wu J, et al. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res Ther. 2020;11(1):224. https://doi.org/10.1186/s13287-020-01737-0.
-
Deng S, Zhou X, Ge Z, Song Y, Wang H, Liu X, et al. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int J Biochem Cell Biol. 2019;114: 105564. https://doi.org/10.1016/j.biocel.2019.105564.
-
Zhang CS, Shao K, Liu CW, Li CJ, Yu BT. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur Rev Med Pharmacol Sci. 2019;23(15):6691–9. https://doi.org/10.26355/eurrev_201908_18560.
-
Chen Q, Liu Y, Ding X, Li Q, Qiu F, Wang M, et al. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol Cell Biochem. 2020;465(1–2):103–14. https://doi.org/10.1007/s11010-019-03671-z.
-
Luo Q, Guo D, Liu G, Chen G, Hang M, Jin M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell Physiol Biochem. 2017;44(6):2105–16. https://doi.org/10.1159/000485949.
-
Tan SJO, Floriano JF, Nicastro L, Emanueli C, Catapano F. Novel Applications of Mesenchymal Stem Cell-derived Exosomes for Myocardial Infarction Therapeutics. Biomolecules. 2020;10(5). https://doi.org/10.3390/biom10050707.
-
Eble JA, Niland S. The extracellular matrix of blood vessels. Curr Pharm Des. 2009;15(12):1385–400. https://doi.org/10.2174/138161209787846757.
-
Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–27. https://doi.org/10.1038/nrd3455.
-
De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol. 2009;29(5):639–49. https://doi.org/10.1161/atvbaha.109.185165.
-
Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146(6):873–87. https://doi.org/10.1016/j.cell.2011.08.039.
-
Ribatti D, Crivellato E. “Sprouting angiogenesis”, a reappraisal. Dev Biol. 2012;372(2):157–65. https://doi.org/10.1016/j.ydbio.2012.09.018.
-
Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143(2):181–96. https://doi.org/10.1016/j.pharmthera.2014.02.013.
-
Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, et al. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91(3):402–11. https://doi.org/10.1093/cvr/cvr053.
-
Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377–84. https://doi.org/10.1634/stemcells.22-3-377.
-
Hao L, Sun H, Wang J, Wang T, Wang M, Zou Z. Mesenchymal stromal cells for cell therapy: besides supporting hematopoiesis. Int J Hematol. 2012;95(1):34–46. https://doi.org/10.1007/s12185-011-0991-8.
-
Kheirandish M, Gavgani SP, Samiee S. The effect of hypoxia preconditioning on the neural and stemness genes expression profiling in human umbilical cord blood mesenchymal stem cells. Transfus Apher Sci. 2017;56(3):392–9. https://doi.org/10.1016/j.transci.2017.03.015.
-
Kim YS, Noh MY, Cho KA, Kim H, Kwon MS, Kim KS, et al. Hypoxia/Reoxygenation-Preconditioned Human Bone Marrow-Derived Mesenchymal Stromal Cells Rescue Ischemic Rat Cortical Neurons by Enhancing Trophic Factor Release. Mol Neurobiol. 2015;52(1):792–803. https://doi.org/10.1007/s12035-014-8912-5.
-
Hu X, Wei L, Taylor TM, Wei J, Zhou X, Wang JA, et al. Hypoxic preconditioning enhances bone marrow mesenchymal stem cell migration via Kv2.1 channel and FAK activation. Am J Physiol Cell Physiol. 2011;301(2):C362–72. https://doi.org/10.1152/ajpcell.00013.2010.
-
Lu F, Zhao X, Wu J, Cui Y, Mao Y, Chen K, et al. MSCs transfected with hepatocyte growth factor or vascular endothelial growth factor improve cardiac function in the infarcted porcine heart by increasing angiogenesis and reducing fibrosis. Int J Cardiol. 2013;167(6):2524–32. https://doi.org/10.1016/j.ijcard.2012.06.052.
-
Sun L, Cui M, Wang Z, Feng X, Mao J, Chen P, et al. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem Biophys Res Commun. 2007;357(3):779–84. https://doi.org/10.1016/j.bbrc.2007.04.010.
-
Schmidt A, Ladage D, Schinköthe T, Klausmann U, Ulrichs C, Klinz FJ, et al. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells. 2006;24(7):1750–8. https://doi.org/10.1634/stemcells.2005-0191.
-
Langer HF, Stellos K, Steingen C, Froihofer A, Schönberger T, Krämer B, et al. Platelet derived bFGF mediates vascular integrative mechanisms of mesenchymal stem cells in vitro. J Mol Cell Cardiol. 2009;47(2):315–25. https://doi.org/10.1016/j.yjmcc.2009.03.011.
-
Miyamoto K, Yazawa T, Mizutani T, Imamichi Y, Kawabe SY, Kanno M, et al. Stem cell differentiation into steroidogenic cell lineages by NR5A family. Mol Cell Endocrinol. 2011;336(1–2):123–6. https://doi.org/10.1016/j.mce.2010.11.031.
-
Park S, Choi Y, Jung N, Yu Y, Ryu KH, Kim HS, et al. Myogenic differentiation potential of human tonsil-derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int J Mol Med. 2016;37(5):1209–20. https://doi.org/10.3892/ijmm.2016.2536.
-
Yu Y, Huang H, Ye J, Li Y, Xie R, Zeng L, et al. 3D Spheroids Facilitate Differentiation of Human Adipose-Derived Mesenchymal Stem Cells into Hepatocyte-Like Cells via p300-Mediated H3K56 Acetylation. Stem Cells Transl Med. 2024;13(2):151–65. https://doi.org/10.1093/stcltm/szad076.
-
Gerami MH, Khorram R, Rasoolzadegan S, Mardpour S, Nakhaei P, Hashemi S, et al. Emerging role of mesenchymal stem/stromal cells (MSCs) and MSCs-derived exosomes in bone- and joint-associated musculoskeletal disorders: a new frontier. Eur J Med Res. 2023;28(1):86. https://doi.org/10.1186/s40001-023-01034-5.
-
Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–6. https://doi.org/10.1161/01.Cir.0000151812.86142.45.
-
Alviano F, Fossati V, Marchionni C, Arpinati M, Bonsi L, Franchina M, et al. Term Amniotic membrane is a high throughput source for multipotent Mesenchymal Stem Cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11. https://doi.org/10.1186/1471-213x-7-11.
-
Vittorio O, Jacchetti E, Pacini S, Cecchini M. Endothelial differentiation of mesenchymal stromal cells: when traditional biology meets mechanotransduction. Integr Biol (Camb). 2013;5(2):291–9. https://doi.org/10.1039/c2ib20152f.
-
Shafei AE, Ali MA, Ghanem HG, Shehata AI, Abdelgawad AA, Handal HR, et al. Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction. J Gene Med. 2017;19(12). https://doi.org/10.1002/jgm.2995.
-
Pati S, Khakoo AY, Zhao J, Jimenez F, Gerber MH, Harting M, et al. Human mesenchymal stem cells inhibit vascular permeability by modulating vascular endothelial cadherin/β-catenin signaling. Stem Cells Dev. 2011;20(1):89–101. https://doi.org/10.1089/scd.2010.0013.
-
Liu K, Ji K, Guo L, Wu W, Lu H, Shan P, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res. 2014;92:10–8. https://doi.org/10.1016/j.mvr.2014.01.008.
-
Hu X, Ning X, Zhao Q, Zhang Z, Zhang C, Xie M, et al. Islet-1 Mesenchymal Stem Cells-Derived Exosome-Incorporated Angiogenin-1 Hydrogel for Enhanced Acute Myocardial Infarction Therapy. ACS Appl Mater Interfaces. 2022;14(32):36289–303. https://doi.org/10.1021/acsami.2c04686.
-
Lee JR, Park BW, Kim J, Choo YW, Kim HY, Yoon JK, et al. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci Adv. 2020;6(18):eaaz0952. https://doi.org/10.1126/sciadv.aaz0952.
-
Kang K, Ma R, Cai W, Huang W, Paul C, Liang J, et al. Exosomes Secreted from CXCR4 Overexpressing Mesenchymal Stem Cells Promote Cardioprotection via Akt Signaling Pathway following Myocardial Infarction. Stem Cells Int. 2015;2015: 659890. https://doi.org/10.1155/2015/659890.
-
Huang P, Wang L, Li Q, Tian X, Xu J, Xu J, et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res. 2020;116(2):353–67. https://doi.org/10.1093/cvr/cvz139.
-
Zhu D, Liu S, Huang K, Wang Z, Hu S, Li J, et al. Intrapericardial Exosome Therapy Dampens Cardiac Injury via Activating Foxo3. Circ Res. 2022;131(10):e135–50. https://doi.org/10.1161/circresaha.122.321384.
-
Yao J, Huang K, Zhu D, Chen T, Jiang Y, Zhang J, et al. A Minimally Invasive Exosome Spray Repairs Heart after Myocardial Infarction. ACS Nano. 2021;15(7):11099–111. https://doi.org/10.1021/acsnano.1c00628.
-
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010.
-
Shi HT, Huang ZH, Xu TZ, Sun AJ, Ge JB. New diagnostic and therapeutic strategies for myocardial infarction via nanomaterials. EBioMedicine. 2022;78: 103968. https://doi.org/10.1016/j.ebiom.2022.103968.
-
Piamsiri C, Maneechote C, Siri-Angkul N, Chattipakorn SC, Chattipakorn N. Targeting necroptosis as therapeutic potential in chronic myocardial infarction. J Biomed Sci. 2021;28(1):25. https://doi.org/10.1186/s12929-021-00722-w.
-
Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116(12):1887–906. https://doi.org/10.1161/circresaha.116.304521.
-
Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106(15):1913–8. https://doi.org/10.1161/01.cir.0000034046.87607.1c.
-
Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94(1):92–5. https://doi.org/10.1016/j.amjcard.2004.03.034.
-
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–86. https://doi.org/10.1016/j.jacc.2009.06.055.
-
Kim SH, Cho JH, Lee YH, Lee JH, Kim SS, Kim MY, et al. Improvement in Left Ventricular Function with Intracoronary Mesenchymal Stem Cell Therapy in a Patient with Anterior Wall ST-Segment Elevation Myocardial Infarction. Cardiovasc Drugs Ther. 2018;32(4):329–38. https://doi.org/10.1007/s10557-018-6804-z.
-
Wollert KC, Meyer GP, Müller-Ehmsen J, Tschöpe C, Bonarjee V, Larsen AI, et al. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST-2 randomised placebo-controlled clinical trial. Eur Heart J. 2017;38(39):2936–43. https://doi.org/10.1093/eurheartj/ehx188.
-
Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–209. https://doi.org/10.1056/NEJMoa055706.
-
Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Kofoed KF, Haack-Sørensen M, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail. 2020;22(5):884–92. https://doi.org/10.1002/ejhf.1700.
-
Bolli R, Kahlon A. Time to end the war on cell therapy. Eur J Heart Fail. 2020;22(5):893–7. https://doi.org/10.1002/ejhf.1767.
-
Attar A, Farjoud Kouhanjani M, Hessami K, Vosough M, Kojuri J, Ramzi M, et al. Effect of once versus twice intracoronary injection of allogeneic-derived mesenchymal stromal cells after acute myocardial infarction: BOOSTER-TAHA7 randomized clinical trial. Stem Cell Res Ther. 2023;14(1):264. https://doi.org/10.1186/s13287-023-03495-1.
-
Zhang R, Yu J, Zhang N, Li W, Wang J, Cai G, et al. Bone marrow mesenchymal stem cells transfer in patients with ST-segment elevation myocardial infarction: single-blind, multicenter, randomized controlled trial. Stem Cell Res Ther. 2021;12(1):33. https://doi.org/10.1186/s13287-020-02096-6.
-
Lee JW, Lee SH, Youn YJ, Ahn MS, Kim JY, Yoo BS, et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014;29(1):23–31. https://doi.org/10.3346/jkms.2014.29.1.23.
-
Gao LR, Chen Y, Zhang NK, Yang XL, Liu HL, Wang ZG, et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BMC Med. 2015;13:162. https://doi.org/10.1186/s12916-015-0399-z.
-
Chullikana A, Majumdar AS, Gottipamula S, Krishnamurthy S, Kumar AS, Prakash VS, et al. Randomized, double-blind, phase I/II study of intravenous allogeneic mesenchymal stromal cells in acute myocardial infarction. Cytotherapy. 2015;17(3):250–61. https://doi.org/10.1016/j.jcyt.2014.10.009.
-
Chen SL, Fang WW, Qian J, Ye F, Liu YH, Shan SJ, et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl). 2004;117(10):1443–8.
-
Mohyeddin-Bonab M, Mohamad-Hassani MR, Alimoghaddam K, Sanatkar M, Gasemi M, Mirkhani H, et al. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med. 2007;10(4):467–73.
-
Gao LR, Pei XT, Ding QA, Chen Y, Zhang NK, Chen HY, et al. A critical challenge: dosage-related efficacy and acute complication intracoronary injection of autologous bone marrow mesenchymal stem cells in acute myocardial infarction. Int J Cardiol. 2013;168(4):3191–9. https://doi.org/10.1016/j.ijcard.2013.04.112.
-
Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311(1):62–73. https://doi.org/10.1001/jama.2013.282909.
-
Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA, et al. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65(3):321–9. https://doi.org/10.1002/ccd.20406.
-
Rodrigo SF, van Ramshorst J, Hoogslag GE, Boden H, Velders MA, Cannegieter SC, et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res. 2013;6(5):816–25. https://doi.org/10.1007/s12265-013-9507-7.
-
Yang Z, Zhang F, Ma W, Chen B, Zhou F, Xu Z, et al. A novel approach to transplanting bone marrow stem cells to repair human myocardial infarction: delivery via a noninfarct-relative artery. Cardiovasc Ther. 2010;28(6):380–5. https://doi.org/10.1111/j.1755-5922.2009.00116.x.
-
Wang X, Xi WC, Wang F. The beneficial effects of intracoronary autologous bone marrow stem cell transfer as an adjunct to percutaneous coronary intervention in patients with acute myocardial infarction. Biotechnol Lett. 2014;36(11):2163–8. https://doi.org/10.1007/s10529-014-1589-z.
-
Choudry F, Hamshere S, Saunders N, Veerapen J, Bavnbek K, Knight C, et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trial†. Eur Heart J. 2016;37(3):256–63. https://doi.org/10.1093/eurheartj/ehv493.
-
Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012;59(5):539–40. https://doi.org/10.1016/j.jacc.2011.09.065.
-
Wöhrle J, von Scheidt F, Schauwecker P, Wiesneth M, Markovic S, Schrezenmeier H, et al. Impact of cell number and microvascular obstruction in patients with bone-marrow derived cell therapy: final results from the randomized, double-blind, placebo controlled intracoronary Stem Cell therapy in patients with Acute Myocardial Infarction (SCAMI) trial. Clin Res Cardiol. 2013;102(10):765–70. https://doi.org/10.1007/s00392-013-0595-9.
-
Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–21. https://doi.org/10.1056/NEJMoa060186.
-
Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–21. https://doi.org/10.1016/s0140-6736(05)67861-0.
-
Benedek I, Bucur O, Benedek T. Intracoronary infusion of mononuclear bone marrow-derived stem cells is associated with a lower plaque burden after four years. J Atheroscler Thromb. 2014;21(3):217–29. https://doi.org/10.5551/jat.19745.
-
Peregud-Pogorzelska M, Przybycień K, Baumert B, Kotowski M, Pius-Sadowska E, Safranow K, et al. The Effect of Intracoronary Infusion of Autologous Bone Marrow-Derived Lineage-Negative Stem/Progenitor Cells on Remodeling of Post-Infarcted Heart in Patient with Acute Myocardial Infarction. Int J Med Sci. 2020;17(8):985–94. https://doi.org/10.7150/ijms.42561.
-
Hopp E, Lunde K, Solheim S, Aakhus S, Arnesen H, Forfang K, et al. Regional myocardial function after intracoronary bone marrow cell injection in reperfused anterior wall infarction – a cardiovascular magnetic resonance tagging study. J Cardiovasc Magn Reson. 2011;13(1):22. https://doi.org/10.1186/1532-429x-13-22.
-
Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–94. https://doi.org/10.1161/circulationaha.105.575118.
-
Lunde K, Solheim S, Aakhus S, Arnesen H, Moum T, Abdelnoor M, et al. Exercise capacity and quality of life after intracoronary injection of autologous mononuclear bone marrow cells in acute myocardial infarction: results from the Autologous Stem cell Transplantation in Acute Myocardial Infarction (ASTAMI) randomized controlled trial. Am Heart J. 2007;154(4):710.e1-8. https://doi.org/10.1016/j.ahj.2007.07.003.
-
Golpanian S, El-Khorazaty J, Mendizabal A, DiFede DL, Suncion VY, Karantalis V, et al. Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J Am Coll Cardiol. 2015;65(2):125–32. https://doi.org/10.1016/j.jacc.2014.10.040.
-
Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, et al. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol. 2014;63(2):110–22. https://doi.org/10.1016/j.jacc.2013.08.724.
-
Florea V, Rieger AC, DiFede DL, El-Khorazaty J, Natsumeda M, Banerjee MN, et al. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study). Circ Res. 2017;121(11):1279–90. https://doi.org/10.1161/circresaha.117.311827.
-
Suncion VY, Ghersin E, Fishman JE, Zambrano JP, Karantalis V, Mandel N, et al. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally?: An analysis from the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis (POSEIDON) randomized trial. Circ Res. 2014;114(8):1292–301. https://doi.org/10.1161/circresaha.114.302854.
-
Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308(22):2369–79. https://doi.org/10.1001/jama.2012.25321.
-
Hsiao LC, Lin YN, Shyu WC, Ho M, Lu CR, Chang SS, et al. First-in-human pilot trial of combined intracoronary and intravenous mesenchymal stem cell therapy in acute myocardial infarction. Front Cardiovasc Med. 2022;9: 961920. https://doi.org/10.3389/fcvm.2022.961920.
-
Li X, Hu YD, Guo Y, Chen Y, Guo DX, Zhou HL, et al. Safety and efficacy of intracoronary human umbilical cord-derived mesenchymal stem cell treatment for very old patients with coronary chronic total occlusion. Curr Pharm Des. 2015;21(11):1426–32. https://doi.org/10.2174/1381612821666141126100636.
-
Prat-Vidal C, Rodríguez-Gómez L, Aylagas M, Nieto-Nicolau N, Gastelurrutia P, Agustí E, et al. First-in-human PeriCord cardiac bioimplant: Scalability and GMP manufacturing of an allogeneic engineered tissue graft. EBioMedicine. 2020;54: 102729. https://doi.org/10.1016/j.ebiom.2020.102729.
-
Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108(6):2114–20. https://doi.org/10.1182/blood-2005-11-011650.
-
Harrell CR, Jovicic N, Djonov V, Volarevic V. Therapeutic Use of Mesenchymal Stem Cell-Derived Exosomes: From Basic Science to Clinics. Pharmaceutics. 2020;12(5). https://doi.org/10.3390/pharmaceutics12050474.
-
J D, Y S, S Z, L C, B L, J Y, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal transduction and targeted therapy. 2020;5(1):145. https://doi.org/10.1038/s41392-020-00261-0.
-
Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet (London, England). 2024;403(10440):2133–61. https://doi.org/10.1016/s0140-6736(24)00757-8.
-
Gupta A, Vardalakis N, Wagner FB. Neuroprosthetics: from sensorimotor to cognitive disorders. Commun Biol. 2023;6(1):14. https://doi.org/10.1038/s42003-022-04390-w.
-
Namini MS, Daneshimehr F, Beheshtizadeh N, Mansouri V, Ai J, Jahromi HK, et al. Cell-free therapy based on extracellular vesicles: a promising therapeutic strategy for peripheral nerve injury. Stem Cell Res Ther. 2023;14(1):254. https://doi.org/10.1186/s13287-023-03467-5.
-
Mercuri E, Sumner CJ, Muntoni F, Darras BT, Finkel RS. Spinal muscular atrophy. Nat Rev Dis Primers. 2022;8(1):52. https://doi.org/10.1038/s41572-022-00380-8.
-
El-Badawy A, Amer M, Abdelbaset R, Sherif SN, Abo-Elela M, Ghallab YH, et al. Adipose Stem Cells Display Higher Regenerative Capacities and More Adaptable Electro-Kinetic Properties Compared to Bone Marrow-Derived Mesenchymal Stromal Cells. Sci Rep. 2016;6:37801. https://doi.org/10.1038/srep37801.
-
Tögel F, Yang Y, Zhang P, Hu Z, Westenfelder C. Bioluminescence imaging to monitor the in vivo distribution of administered mesenchymal stem cells in acute kidney injury. Am J Physiol Renal Physiol. 2008;295(1):F315–21. https://doi.org/10.1152/ajprenal.00098.2008.
-
Mohseni R, Hamidieh AA, Shoae-Hassani A, Ghahvechi-Akbari M, Majma A, Mohammadi M, et al. An open-label phase 1 clinical trial of the allogeneic side population adipose-derived mesenchymal stem cells in SMA type 1 patients. Neurol Sci. 2022;43(1):399–410. https://doi.org/10.1007/s10072-021-05291-2.
-
Harris VK, Stark J, Williams A, Roche M, Malin M, Kumar A, et al. Efficacy of intrathecal mesenchymal stem cell-neural progenitor therapy in progressive MS: results from a phase II, randomized, placebo-controlled clinical trial. Stem Cell Res Ther. 2024;15(1):151. https://doi.org/10.1186/s13287-024-03765-6.
-
Sung Y, Lee SM, Park M, Choi HJ, Kang S, Choi BI, et al. Treatment of traumatic optic neuropathy using human placenta-derived mesenchymal stem cells in Asian patients. Regen Med. 2020;15(10):2163–79. https://doi.org/10.2217/rme-2020-0044.
-
Tremblay F, Ansari Y, Remaud A, Freedman MS. Neurophysiological outcomes following mesenchymal stem cell therapy in multiple sclerosis. Clin Neurophysiol. 2022;136:69–81. https://doi.org/10.1016/j.clinph.2022.01.125.
-
Cotten CM, Fisher K, Malcolm W, Gustafson KE, Cheatham L, Marion A, et al. A Pilot Phase I Trial of Allogeneic Umbilical Cord Tissue-Derived Mesenchymal Stromal Cells in Neonates With Hypoxic-Ischemic Encephalopathy. Stem Cells Transl Med. 2023;12(6):355–64. https://doi.org/10.1093/stcltm/szad027.
-
Petrou P, Kassis I, Ginzberg A, Hallimi M, Karussis D. Effects of Mesenchymal Stem Cell Transplantation on Cerebrospinal Fluid Biomarkers in Progressive Multiple Sclerosis. Stem Cells Transl Med. 2022;11(1):55–8. https://doi.org/10.1093/stcltm/szab017.
-
Özmert E, Arslan U. Management of toxic optic neuropathy via a combination of Wharton’s jelly-derived mesenchymal stem cells with electromagnetic stimulation. Stem Cell Res Ther. 2021;12(1):518. https://doi.org/10.1186/s13287-021-02577-2.
-
Siwek T, Jezierska-Woźniak K, Maksymowicz S, Barczewska M, Sowa M, Badowska W, et al. Repeat Administration of Bone Marrow-Derived Mesenchymal Stem Cells for Treatment of Amyotrophic Lateral Sclerosis. Med Sci Monit. 2020;26: e927484. https://doi.org/10.12659/msm.927484.
-
Awidi A, Al Shudifat A, El Adwan N, Alqudah M, Jamali F, Nazer F, et al. Safety and potential efficacy of expanded mesenchymal stromal cells of bone marrow and umbilical cord origins in patients with chronic spinal cord injuries: a phase I/II study. Cytotherapy. 2024;26(8):825–31. https://doi.org/10.1016/j.jcyt.2024.03.480.
-
Albu S, Kumru H, Coll R, Vives J, Vallés M, Benito-Penalva J, et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: a randomized controlled study. Cytotherapy. 2021;23(2):146–56. https://doi.org/10.1016/j.jcyt.2020.08.008.
-
Zamani H, Soufizomorrod M, Oraee-Yazdani S, Naviafar D, Akhlaghpasand M, Seddighi A, et al. Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: a clinical trial. Spinal Cord. 2022;60(1):63–70. https://doi.org/10.1038/s41393-021-00687-5.
-
Li J, Bai X, Guan X, Yuan H, Xu X. Treatment of Optic Canal Decompression Combined with Umbilical Cord Mesenchymal Stem (Stromal) Cells for Indirect Traumatic Optic Neuropathy: A Phase 1 Clinical Trial. Ophthalmic Res. 2021;64(3):398–404. https://doi.org/10.1159/000512469.
-
Law ZK, Tan HJ, Chin SP, Wong CY, Wan Yahya WNN, Muda AS, et al. The effects of intravenous infusion of autologous mesenchymal stromal cells in patients with subacute middle cerebral artery infarct: a phase 2 randomized controlled trial on safety, tolerability and efficacy. Cytotherapy. 2021;23(9):833–40. https://doi.org/10.1016/j.jcyt.2021.03.005.
-
Gu J, Huang L, Zhang C, Wang Y, Zhang R, Tu Z, et al. Therapeutic evidence of umbilical cord-derived mesenchymal stem cell transplantation for cerebral palsy: a randomized, controlled trial. Stem Cell Res Ther. 2020;11(1):43. https://doi.org/10.1186/s13287-019-1545-x.
-
Chung JW, Chang WH, Bang OY, Moon GJ, Kim SJ, Kim SK, et al. Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology. 2021;96(7):e1012–23. https://doi.org/10.1212/wnl.0000000000011440.
-
Zhuo Y, Li WS, Lu W, Li X, Ge LT, Huang Y, et al. TGF-β1 mediates hypoxia-preconditioned olfactory mucosa mesenchymal stem cells improved neural functional recovery in Parkinson’s disease models and patients. Mil Med Res. 2024;11(1):48. https://doi.org/10.1186/s40779-024-00550-7.
-
Baak LM, Wagenaar N, van der Aa NE, Groenendaal F, Dudink J, Tataranno ML, et al. Feasibility and safety of intranasally administered mesenchymal stromal cells after perinatal arterial ischaemic stroke in the Netherlands (PASSIoN): a first-in-human, open-label intervention study. Lancet Neurol. 2022;21(6):528–36. https://doi.org/10.1016/s1474-4422(22)00117-x.
-
Bydon M, Qu W, Moinuddin FM, Hunt CL, Garlanger KL, Reeves RK, et al. Intrathecal delivery of adipose-derived mesenchymal stem cells in traumatic spinal cord injury: Phase I trial. Nat Commun. 2024;15(1):2201. https://doi.org/10.1038/s41467-024-46259-y.
-
Kim HJ, Cho KR, Jang H, Lee NK, Jung YH, Kim JP, et al. Intracerebroventricular injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase I clinical trial. Alzheimers Res Ther. 2021;13(1):154. https://doi.org/10.1186/s13195-021-00897-2.
-
Cudkowicz ME, Lindborg SR, Goyal NA, Miller RG, Burford MJ, Berry JD, et al. A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve. 2022;65(3):291–302. https://doi.org/10.1002/mus.27472.
-
Uccelli A, Laroni A, Ali R, Battaglia MA, Blinkenberg M, Brundin L, et al. Safety, tolerability, and activity of mesenchymal stem cells versus placebo in multiple sclerosis (MESEMS): a phase 2, randomised, double-blind crossover trial. Lancet Neurol. 2021;20(11):917–29. https://doi.org/10.1016/s1474-4422(21)00301-x.
-
Pastor JC, Pastor-Idoate S, López-Paniagua M, Para M, Blazquez F, Murgui E, et al. Intravitreal allogeneic mesenchymal stem cells: a non-randomized phase II clinical trial for acute non-arteritic optic neuropathy. Stem Cell Res Ther. 2023;14(1):261. https://doi.org/10.1186/s13287-023-03500-7.
-
Lee J, Chang WH, Chung JW, Kim SJ, Kim SK, Lee JS, et al. Efficacy of Intravenous Mesenchymal Stem Cells for Motor Recovery After Ischemic Stroke: A Neuroimaging Study. Stroke. 2022;53(1):20–8. https://doi.org/10.1161/strokeaha.121.034505.
-
Jamali F, Aldughmi M, Atiani S, Al-Radaideh A, Dahbour S, Alhattab D, et al. Human Umbilical Cord-Derived Mesenchymal Stem Cells in the Treatment of Multiple Sclerosis Patients: Phase I/II Dose-Finding Clinical Study. Cell Transplant. 2024;33:9636897241233044. https://doi.org/10.1177/09636897241233045.
-
Amanat M, Majmaa A, Zarrabi M, Nouri M, Akbari MG, Moaiedi AR, et al. Clinical and imaging outcomes after intrathecal injection of umbilical cord tissue mesenchymal stem cells in cerebral palsy: a randomized double-blind sham-controlled clinical trial. Stem Cell Res Ther. 2021;12(1):439. https://doi.org/10.1186/s13287-021-02513-4.
-
Sun JM, Case LE, McLaughlin C, Burgess A, Skergan N, Crane S, et al. Motor function and safety after allogeneic cord blood and cord tissue-derived mesenchymal stromal cells in cerebral palsy: An open-label, randomized trial. Dev Med Child Neurol. 2022;64(12):1477–86. https://doi.org/10.1111/dmcn.15325.
-
Akhlaghpasand M, Tavanaei R, Hosseinpoor M, Yazdani KO, Soleimani A, Zoshk MY, et al. Safety and potential effects of intrathecal injection of allogeneic human umbilical cord mesenchymal stem cell-derived exosomes in complete subacute spinal cord injury: a first-in-human, single-arm, open-label, phase I clinical trial. Stem Cell Res Ther. 2024;15(1):264. https://doi.org/10.1186/s13287-024-03868-0.
-
Cohen JA, Lublin FD, Lock C, Pelletier D, Chitnis T, Mehra M, et al. Evaluation of neurotrophic factor secreting mesenchymal stem cells in progressive multiple sclerosis. Mult Scler. 2023;29(1):92–106. https://doi.org/10.1177/13524585221122156.
-
Barczewska M, Maksymowicz S, Zdolińska-Malinowska I, Siwek T, Grudniak M. Umbilical Cord Mesenchymal Stem Cells in Amyotrophic Lateral Sclerosis: an Original Study. Stem Cell Rev Rep. 2020;16(5):922–32. https://doi.org/10.1007/s12015-020-10016-7.
-
Bang OY, Kim EH, Cho YH, Oh MJ, Chung JW, Chang WH, et al. Circulating Extracellular Vesicles in Stroke Patients Treated With Mesenchymal Stem Cells: A Biomarker Analysis of a Randomized Trial. Stroke. 2022;53(7):2276–86. https://doi.org/10.1161/strokeaha.121.036545.
-
de Celis-Ruiz E, Fuentes B, Alonso de Leciñana M, Gutiérrez-Fernández M, Borobia AM, Gutiérrez-Zúñiga R, et al. Final Results of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells in Acute Ischemic Stroke (AMASCIS): A Phase II, Randomized, Double-Blind, Placebo-Controlled, Single-Center, Pilot Clinical Trial. Cell Transplant. 2022;31:9636897221083863. https://doi.org/10.1177/09636897221083863.
-
Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M, et al. Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: a Randomized Clinical Trial. Transl Stroke Res. 2020;11(5):910–23. https://doi.org/10.1007/s12975-020-00787-z.
-
Petrou P, Kassis I, Yaghmour NE, Ginzberg A, Karussis D. A phase II clinical trial with repeated intrathecal injections of autologous mesenchymal stem cells in patients with amyotrophic lateral sclerosis. Front Biosci (Landmark Ed). 2021;26(10):693–706. https://doi.org/10.52586/4980.
-
Lu Z, Zhu L, Liu Z, Wu J, Xu Y, Zhang CJ. IV/IT hUC-MSCs Infusion in RRMS and NMO: A 10-Year Follow-Up Study. Front Neurol. 2020;11:967. https://doi.org/10.3389/fneur.2020.00967.
-
Lai B, Wu CH, Wu CY, Luo SF, Lai JH. Ferroptosis and Autoimmune Diseases Front Immunol. 2022;13: 916664. https://doi.org/10.3389/fimmu.2022.916664.
-
Zx X, Js M, Sg Z. An updated advance of autoantibodies in autoimmune diseases. Autoimmun Rev. 2021;20(2): 102743. https://doi.org/10.1016/j.autrev.2020.102743.
-
Yang C, Sun J, Tian Y, Li H, Zhang L, Yang J, et al. Immunomodulatory Effect of MSCs and MSCs-Derived Extracellular Vesicles in Systemic Lupus Erythematosus. Front Immunol. 2021;12: 714832. https://doi.org/10.3389/fimmu.2021.714832.
-
Nakano M, Nagaishi K, Konari N, Saito Y, Chikenji T, Mizue Y, et al. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. 2016;6:24805. https://doi.org/10.1038/srep24805.
-
Lee DH, Park KS, Shin HE, Kim SB, Choi H, An SB, et al. Safety and Feasibility of Intradiscal Administration of Matrilin-3-Primed Adipose-Derived Mesenchymal Stromal Cell Spheroids for Chronic Discogenic Low Back Pain: Phase 1 Clinical Trial. Int J Mol Sci. 2023;24(23). https://doi.org/10.3390/ijms242316827.
-
Hernigou P, Auregan JC, Dubory A, Flouzat Lachaniette CH, Rouard H. Ankle osteonecrosis in fifty-one children and adolescent’s leukemia survivors: a prospective randomized study on percutaneous mesenchymal stem cells treatment. Int Orthop. 2021;45(9):2383–93. https://doi.org/10.1007/s00264-021-05051-z.
-
Sreeparvathy R, Belludi SA, Prabhu A. Platelet Rich Fibrin Matrix (PRFM) and Peripheral Blood Mesenchymal Stem Cells (PBMSCs) in the management of intraosseous defects – A randomized clinical trial. J Appl Oral Sci. 2024;32: e20230442. https://doi.org/10.1590/1678-7757-2023-0442.
-
Sánchez N, Fierravanti L, Núñez J, Vignoletti F, González-Zamora M, Santamaría S, et al. Periodontal regeneration using a xenogeneic bone substitute seeded with autologous periodontal ligament-derived mesenchymal stem cells: A 12-month quasi-randomized controlled pilot clinical trial. J Clin Periodontol. 2020;47(11):1391–402. https://doi.org/10.1111/jcpe.13368.
-
Infante A, Gener B, Vázquez M, Olivares N, Arrieta A, Grau G, et al. Reiterative infusions of MSCs improve pediatric osteogenesis imperfecta eliciting a pro-osteogenic paracrine response: TERCELOI clinical trial. Clin Transl Med. 2021;11(1): e265. https://doi.org/10.1002/ctm2.265.
-
Hernigou P, Hernigou J, Scarlat M. Mesenchymal stem cell therapy improved outcome of early post-traumatic shoulder osteonecrosis: a prospective randomized clinical study of fifty patients with over ten year follow-up. Int Orthop. 2021;45(10):2643–52. https://doi.org/10.1007/s00264-021-05160-9.
-
Sun H, Zhai H, Han K, Ma H, Tan Y, Li S, et al. Clinical outcomes of autologous adipose-derived mesenchymal stem cell combined with high tibial osteotomy for knee osteoarthritis are correlated with stem cell stemness and senescence. J Transl Med. 2024;22(1):1039. https://doi.org/10.1186/s12967-024-05814-3.
-
Bolandnazar NS, Raeissadat SA, Haghighatkhah H, Rayegani SM, Oshnari RS, Keshel SH, et al. Safety and efficacy of placental mesenchymal stromal cells-derived extracellular vesicles in knee osteoarthritis: a randomized, triple-blind, placebo-controlled clinical trial. BMC Musculoskelet Disord. 2024;25(1):856. https://doi.org/10.1186/s12891-024-07979-w.
-
Matas J, García C, Poblete D, Vernal R, Ortloff A, Luque-Campos N, et al. A Phase I Dose-Escalation Clinical Trial to Assess the Safety and Efficacy of Umbilical Cord-Derived Mesenchymal Stromal Cells in Knee Osteoarthritis. Stem Cells Transl Med. 2024;13(3):193–203. https://doi.org/10.1093/stcltm/szad088.
-
Chen CF, Chen YC, Fu YS, Tsai SW, Wu PK, Chen CM, et al. Safety and Tolerability of Intra-Articular Injection of Adipose-Derived Mesenchymal Stem Cells GXCPC1 in 11 Subjects With Knee Osteoarthritis: A Nonrandomized Pilot Study Without a Control Arm. Cell Transplant. 2024;33:9636897231221882. https://doi.org/10.1177/09636897231221882.
-
Kim KI, Lee MC, Lee JH, Moon YW, Lee WS, Lee HJ, et al. Clinical Efficacy and Safety of the Intra-articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Phase III, Randomized, Double-Blind. Placebo-Controlled Trial Am J Sports Med. 2023;51(9):2243–53. https://doi.org/10.1177/03635465231179223.
-
Sadri B, Hassanzadeh M, Bagherifard A, Mohammadi J, Alikhani M, Moeinabadi-Bidgoli K, et al. Cartilage regeneration and inflammation modulation in knee osteoarthritis following injection of allogeneic adipose-derived mesenchymal stromal cells: a phase II, triple-blinded, placebo controlled, randomized trial. Stem Cell Res Ther. 2023;14(1):162. https://doi.org/10.1186/s13287-023-03359-8.
-
Kim JH, Kim KI, Yoon WK, Song SJ, Jin W. Intra-articular Injection of Mesenchymal Stem Cells After High Tibial Osteotomy in Osteoarthritic Knee: Two-Year Follow-up of Randomized Control Trial. Stem Cells Transl Med. 2022;11(6):572–85. https://doi.org/10.1093/stcltm/szac023.
-
Viganò M, Ragni E, Di Matteo B, Gambaro FM, Perucca Orfei C, Spinelli G, et al. A single step, centrifuge-free method to harvest bone marrow highly concentrated in mesenchymal stem cells: results of a pilot trial. Int Orthop. 2022;46(2):391–400. https://doi.org/10.1007/s00264-021-05243-7.
-
Lamo-Espinosa JM, Blanco JF, Sánchez M, Moreno V, Granero-Moltó F, Sánchez-Guijo F, et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J Transl Med. 2020;18(1):356. https://doi.org/10.1186/s12967-020-02530-6.
-
Bastos R, Mathias M, Andrade R, Amaral R, Schott V, Balduino A, et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc. 2020;28(6):1989–99. https://doi.org/10.1007/s00167-019-05732-8.
-
Vij R, Stebbings KA, Kim H, Park H, Chang D. Safety and efficacy of autologous, adipose-derived mesenchymal stem cells in patients with rheumatoid arthritis: a phase I/IIa, open-label, non-randomized pilot trial. Stem Cell Res Ther. 2022;13(1):88. https://doi.org/10.1186/s13287-022-02763-w.
-
Qi T, Gao H, Dang Y, Huang S, Peng M. Cervus and cucumis peptides combined umbilical cord mesenchymal stem cells therapy for rheumatoid arthritis. Medicine (Baltimore). 2020;99(28): e21222. https://doi.org/10.1097/md.0000000000021222.
-
Kamen DL, Wallace C, Li Z, Wyatt M, Paulos C, Wei C, et al. Safety, immunological effects and clinical response in a phase I trial of umbilical cord mesenchymal stromal cells in patients with treatment refractory SLE. Lupus Sci Med. 2022;9(1). https://doi.org/10.1136/lupus-2022-000704.
-
Fernández O, Izquierdo G, Fernández V, Leyva L, Reyes V, Guerrero M, et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE. 2018;13(5): e0195891. https://doi.org/10.1371/journal.pone.0195891.
-
Chun S, Choi CB, Kim MS, Nam JY, Lee TY, Lee YT, et al. Safety and tolerability of bone marrow-derived mesenchymal stem cells in lupus animal models and a phase I clinical trial in humans. Lupus. 2022;31(10):1245–53. https://doi.org/10.1177/09612033221111957.
-
Alwabli Y, Almatroudi MA, Alharbi MA, Alharbi MY, Alreshood S, Althwiny FA. Work-Related Musculoskeletal Disorders Among Medical Practitioners in the Hospitals of Al’Qassim Region, Saudi Arabia. Cureus. 2020;12(5): e8382. https://doi.org/10.7759/cureus.8382.
-
Chenna D, Pentapati KC, Kumar M, Madi M, Siddiq H. Prevalence of musculoskeletal disorders among dental healthcare providers: A systematic review and meta-analysis. F1000Res. 2022;11:1062. https://doi.org/10.12688/f1000research.124904.2.
-
Kodama J, Wilkinson KJ, Otsuru S. MSC-EV therapy for bone/cartilage diseases. Bone Rep. 2022;17: 101636. https://doi.org/10.1016/j.bonr.2022.101636.
-
Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig. 2019;6:34. https://doi.org/10.21037/sci.2019.08.11.
-
Zhang X, He J, Wang W. Progress in the use of mesenchymal stromal cells for osteoarthritis treatment. Cytotherapy. 2021;23(6):459–70. https://doi.org/10.1016/j.jcyt.2021.01.008.
-
Gilbertie JM, Long JM, Schubert AG, Berglund AK, Schaer TP, Schnabel LV. Pooled Platelet-Rich Plasma Lysate Therapy Increases Synoviocyte Proliferation and Hyaluronic Acid Production While Protecting Chondrocytes From Synoviocyte-Derived Inflammatory Mediators. Front Vet Sci. 2018;5:150. https://doi.org/10.3389/fvets.2018.00150.
-
Chen Y, Xu Y, Chi Y, Sun T, Gao Y, Dou X, et al. Efficacy and safety of human umbilical cord-derived mesenchymal stem cells in the treatment of refractory immune thrombocytopenia: a prospective, single arm, phase I trial. Signal Transduct Target Ther. 2024;9(1):102. https://doi.org/10.1038/s41392-024-01793-5.
-
Carlsson PO, Espes D, Sisay S, Davies LC, Smith CIE, Svahn MG. Umbilical cord-derived mesenchymal stromal cells preserve endogenous insulin production in type 1 diabetes: a Phase I/II randomised double-blind placebo-controlled trial. Diabetologia. 2023;66(8):1431–41. https://doi.org/10.1007/s00125-023-05934-3.
-
Harris VK, Stark JW, Yang S, Zanker S, Tuddenham J, Sadiq SA. Mesenchymal stem cell-derived neural progenitors in progressive MS: Two-year follow-up of a phase I study. Neurol Neuroimmunol Neuroinflamm. 2021;8(1). https://doi.org/10.1212/nxi.0000000000000928.
-
Wu Z, Xu X, Cai J, Chen J, Huang L, Wu W, et al. Prevention of chronic diabetic complications in type 1 diabetes by co-transplantation of umbilical cord mesenchymal stromal cells and autologous bone marrow: a pilot randomized controlled open-label clinical study with 8-year follow-up. Cytotherapy. 2022;24(4):421–7. https://doi.org/10.1016/j.jcyt.2021.09.015.
-
Ma C, Feng Y, Yang L, Wang S, Sun X, Tai S, et al. In vitro Immunomodulatory Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells on Peripheral Blood Cells from Warm Autoimmune Hemolytic Anemia Patients. Acta Haematol. 2022;145(1):63–71. https://doi.org/10.1159/000506759.
-
Lu J, Shen SM, Ling Q, Wang B, Li LR, Zhang W, et al. One repeated transplantation of allogeneic umbilical cord mesenchymal stromal cells in type 1 diabetes: an open parallel controlled clinical study. Stem Cell Res Ther. 2021;12(1):340. https://doi.org/10.1186/s13287-021-02417-3.
-
Wang L, Huang S, Li S, Li M, Shi J, Bai W, et al. Efficacy and Safety of Umbilical Cord Mesenchymal Stem Cell Therapy for Rheumatoid Arthritis Patients: A Prospective Phase I/II Study. Drug Des Devel Ther. 2019;13:4331–40. https://doi.org/10.2147/dddt.S225613.
-
Swart JF, de Roock S, Nievelstein RAJ, Slaper-Cortenbach ICM, Boelens JJ, Wulffraat NM. Bone-marrow derived mesenchymal stromal cells infusion in therapy refractory juvenile idiopathic arthritis patients. Rheumatology (Oxford). 2019;58(10):1812–7. https://doi.org/10.1093/rheumatology/kez157.
-
Ghoryani M, Shariati-Sarabi Z, Tavakkol-Afshari J, Ghasemi A, Poursamimi J, Mohammadi M. Amelioration of clinical symptoms of patients with refractory rheumatoid arthritis following treatment with autologous bone marrow-derived mesenchymal stem cells: A successful clinical trial in Iran. Biomed Pharmacother. 2019;109:1834–40. https://doi.org/10.1016/j.biopha.2018.11.056.
-
Liang J, Zhang H, Kong W, Deng W, Wang D, Feng X, et al. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: a long-term retrospective study. Stem Cell Res Ther. 2018;9(1):312. https://doi.org/10.1186/s13287-018-1053-4.
-
Park EH, Lim HS, Lee S, Roh K, Seo KW, Kang KS, et al. Intravenous Infusion of Umbilical Cord Blood-Derived Mesenchymal Stem Cells in Rheumatoid Arthritis: A Phase Ia Clinical Trial. Stem Cells Transl Med. 2018;7(9):636–42. https://doi.org/10.1002/sctm.18-0031.
-
Ellison-Hughes GM, Colley L, O’Brien KA, Roberts KA, Agbaedeng TA, Ross MD. The Role of MSC Therapy in Attenuating the Damaging Effects of the Cytokine Storm Induced by COVID-19 on the Heart and Cardiovascular System. Front Cardiovasc Med. 2020;7: 602183. https://doi.org/10.3389/fcvm.2020.602183.
-
Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2(12):1016–26. https://doi.org/10.1016/s2213-2600(14)70217-6.
-
Vlahos R, Bozinovski S. Role of alveolar macrophages in chronic obstructive pulmonary disease. Front Immunol. 2014;5:435. https://doi.org/10.3389/fimmu.2014.00435.
-
Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21(10):1199–205. https://doi.org/10.1097/MEG.0b013e32832a1f6c.
-
Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol. 2018;14(6):361–77. https://doi.org/10.1038/s41581-018-0001-y.
-
Barrera-Chimal J, Jaisser F. Pathophysiologic mechanisms in diabetic kidney disease: A focus on current and future therapeutic targets. Diabetes Obes Metab. 2020;22(Suppl 1):16–31. https://doi.org/10.1111/dom.13969.
-
Khalilpourfarshbafi M, Hajiaghaalipour F, Selvarajan KK, Adam A. Mesenchymal Stem Cell-Based Therapies against Podocyte Damage in Diabetic Nephropathy. Tissue Eng Regen Med. 2017;14(3):201–10. https://doi.org/10.1007/s13770-017-0026-5.
-
Jin J, Qian F, Zheng D, He W, Gong J, He Q. Mesenchymal Stem Cells Attenuate Renal Fibrosis via Exosomes-Mediated Delivery of microRNA Let-7i-5p Antagomir. Int J Nanomedicine. 2021;16:3565–78. https://doi.org/10.2147/ijn.S299969.
-
Yang Y, Wang J, Zhang Y, Hu X, Li L, Chen P. Exosomes derived from mesenchymal stem cells ameliorate renal fibrosis via delivery of miR-186-5p. Hum Cell. 2022;35(1):83–97. https://doi.org/10.1007/s13577-021-00617-w.
-
Vivarelli M, Colucci M, Algeri M, Zotta F, Emma F, L’Erario I, et al. A phase I study of autologous mesenchymal stromal cells for severe steroid-dependent nephrotic syndrome. JCI Insight. 2023;8(18). https://doi.org/10.1172/jci.insight.169424.
-
Perico N, Remuzzi G, Griffin MD, Cockwell P, Maxwell AP, Casiraghi F, et al. Safety and Preliminary Efficacy of Mesenchymal Stromal Cell (ORBCEL-M) Therapy in Diabetic Kidney Disease: A Randomized Clinical Trial (NEPHSTROM). J Am Soc Nephrol. 2023;34(10):1733–51. https://doi.org/10.1681/asn.0000000000000189.
-
Swaminathan M, Kopyt N, Atta MG, Radhakrishnan J, Umanath K, Nguyen S, et al. Pharmacological effects of ex vivo mesenchymal stem cell immunotherapy in patients with acute kidney injury and underlying systemic inflammation. Stem Cells Transl Med. 2021;10(12):1588–601. https://doi.org/10.1002/sctm.21-0043.
-
Abumoawad A, Saad A, Ferguson CM, Eirin A, Herrmann SM, Hickson LJ, et al. In a Phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney Int. 2020;97(4):793–804. https://doi.org/10.1016/j.kint.2019.11.022.
-
J L, H Z, C Z, D W, X M, S Z, et al. Effects of allogeneic mesenchymal stem cell transplantation in the treatment of liver cirrhosis caused by autoimmune diseases. International journal of rheumatic diseases. 2017;20(9):1219–26. https://doi.org/10.1111/1756-185x.13015.
-
Chu M, Wang H, Bian L, Huang J, Wu D, Zhang R, et al. Nebulization Therapy with Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Pneumonia. Stem Cell Rev Rep. 2022;18(6):2152–63. https://doi.org/10.1007/s12015-022-10398-w.
-
Weiss DJ, Segal K, Casaburi R, Hayes J, Tashkin D. Effect of mesenchymal stromal cell infusions on lung function in COPD patients with high CRP levels. Respir Res. 2021;22(1):142. https://doi.org/10.1186/s12931-021-01734-8.
-
Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15(1):39. https://doi.org/10.1186/1465-9921-15-39.
-
Harrell CR, Volarevic A, Djonov V, Volarevic V. Mesenchymal Stem-Cell-Derived Exosomes as Novel Drug Carriers in Anti-Cancer Treatment: A Myth or Reality? Cells. 2025;14(3). https://doi.org/10.3390/cells14030202.
Acknowledgements
We would like to acknowledge the researchers whose relevant works are cited in this review and all co-authors for their support. Additionally, we would like to thank the BioRender research and development team for their support of our drawing.
Funding
This work was supported by the General Project of Science and Technology Department of Shandong Province (ZR2022MH063), the Shandong Province Traditional Chinese Medicine Science and Technology Development Plan Project (Q2022122), the Science and Technology Program of Binzhou Medical University Hospital (BY2021 KYQD36), and the Science Research Initiation Project of Binzhou Medical University Hospital (2023–03).
Author information
- Yang Wang and Huanhui Wang should be considered joint first author.
Authors and Affiliations
-
Department of Rehabilitation, Binzhou Medical University Hospital, Binzhou, China
Yang Wang, Zhijie Cao, Qun Wang, Hongkun Wang & Wei Li
-
Binzhou Medical University, Yantai, China
Huanhui Wang
-
Department of Ultrasound Medicine, Binzhou People’s Hospital, Binzhou, China
Jing Tan
-
Department of Physical Medicine and Rehabilitation, Qilu Hospital, Medical School of Shandong University, Jinan, China
Yang Wang & Shouwei Yue
-
Department of Cardiology, Binzhou Medical University Hospital, Binzhou, China
Dong Wang
Contributions
Yang Wang wrote the manuscript. Huanhui Wang constructed the figures and table. Jing Tan and Zhijie Cao contributed to the literature search. Qun Wang, Hongkun Wang and Shouwei Yue reviewed the manuscript. Wei Li and Dong Wang contributed to the review and editing of the manuscript prior to submission. All authors have read and approved the final version of the manuscript.